Rheotaxis facilitates upstream navigation of mammalian sperm cells
Figures
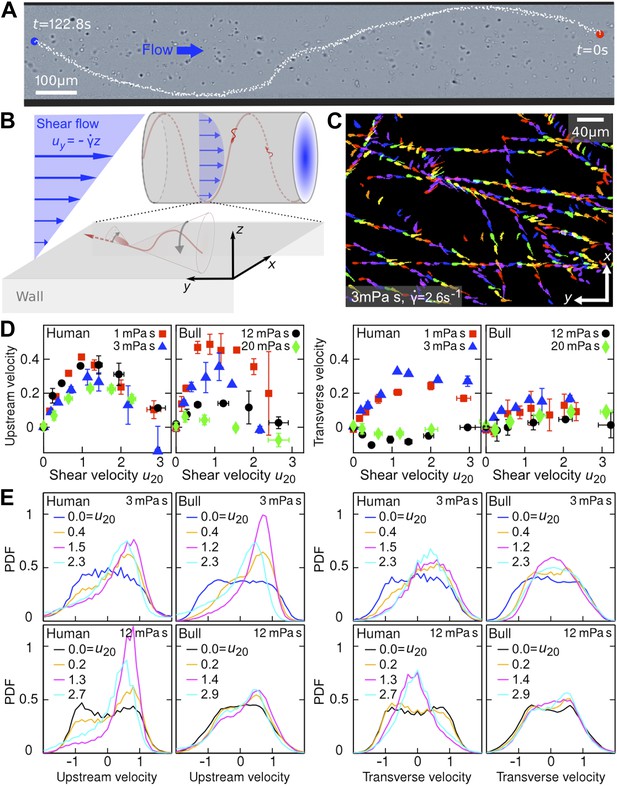
Sperm swim on upstream spirals against shear flow.
(A) Background-subtracted micrograph showing the track of a bull sperm in a cylindrical channel (viscosity μ = 3 mPa·s shear rate ), channel boundary false-coloured with black, see Video 1 for raw data. (B) Schematic representation not drawn to scale. The conical envelope of the flagellar beat holds the sperm close to the surface (Kantsler et al., 2013). The vertical flow gradient exerts a torque that turns the sperm against the flow, but is counteracted by a torque from the chirality of the flagellar wave, resulting in a mean diagonal upstream motion. (C) Tracks of bull sperm near a flat channel surface. (D) Upstream and transverse mean velocities vs shear flow speed u20 at 20 μm from the surface for different viscosities. All velocities are normalised by the sample mean speed v0μ at . For human sperm, in order of increasing viscosity v0μ = 53.5 ± 3.0, 46.8 ± 3.7, 36.8 ± 3.3, 29.7 ± 3.9 μm/s, and for bull sperm v0μ = 70.4 ± 11.8, 45.6 ± 4.7, 32.4 ± 4.8, 29.6 ± 4.1 μm/s, where uncertainties are standard deviations of mean values from different experiments. Each data point is an average over >1000 sperms. (E) Histograms for selected points in (D).
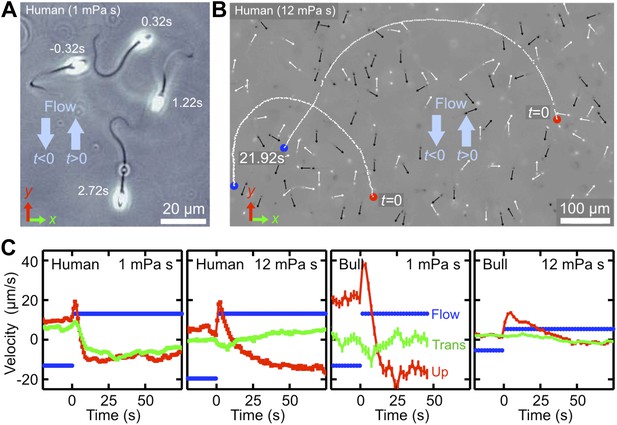
Temporal response of sperm cells to a reversal of the flow direction depends sensitively on viscosity.
(A) At low viscosity, sperm perform sharp U-turns, see also Video 2. (B) At high viscosity, the typical radius of the U-turns increases substantially (Video 3). White/black arrows show orientations of several cells before/after turning. (C) Flow velocity at distance 5 μm from the channel surface (blue, ‘Flow’), mean upstream velocity (red, ‘Up’) and mean transverse velocity (green, ‘Trans’) as function of time. The typical response time of sperm cells after flow reversal increases with viscosity. Peaks reflect a short period when mean swimming direction and flow direction are aligned. The time series for human sperm also signal a suppression of the beat chirality at high viscosity, consistent with Figure 1D.
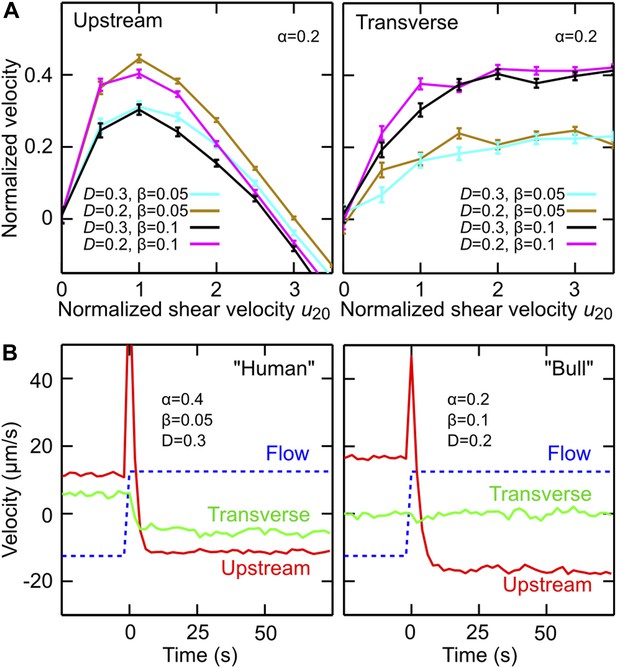
Model simulations reproduce main experimental observations.
(A) Upstream and transverse velocity for different values of the variability (effective noise) parameter D in units rad/s and dimensionless shape factors (α, β). (B) Time response of a chiral swimmer with χ = +1 (‘Human’) and a non-chiral swimmer with χ = 0 (‘Bull’) to a reversal of the flow direction at time t = 0. Blue dashed line shows fluid flow uy at 5 μm from the boundary. Simulation parameters (N = 1000 trajectories, A = 10 μm, ℓ = 60 μm, V = 50 μm/s) were chosen to match approximately those for viscosity 1 mPa·s in Figure 2C.
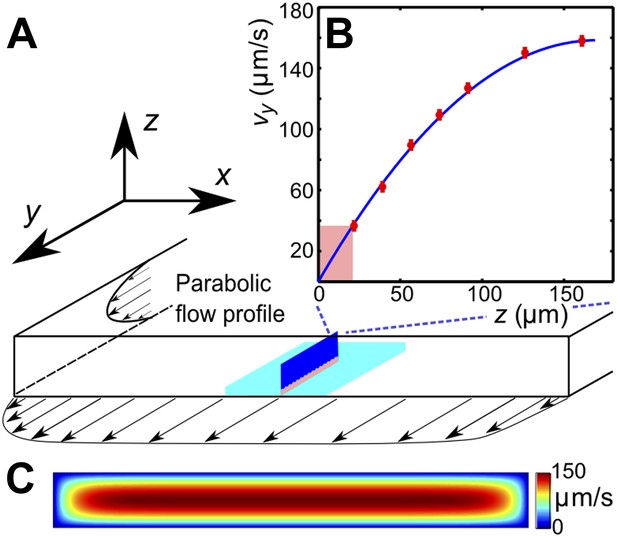
(A) Schematic of the microfluidic channel and field of view (turquoise region) in the sperm motility measurements. (B) Velocity profile at the center of the channel. Red symbols are values of the vertical velocity profile vy(z) measured by PTV for the flow rate 0.1 μl/s. The solid line shows the theoretically calculated flow profile for the same flow rate. In motility experiments, values for the velocity gradient near the boundary (pink region) were obtained by measuring the flow velocity at 20 μm from the boundary. (C) Theoretical 2D flow speed profile in (x,z)-plane at flow rate 0.1 μl/s.
Videos
Human sperm cell swimming on a spiral trajectory (green) against a shear flow in a cylindrical channel (fluid viscosity 3 mPa·s; channel diameter 300 μm; channel boundaries marked in red).
Scale bar 100 μm.
Human sperm cells swimming in a low-viscosity fluid (3 mPa·s) near the wall of a planar channel.
The video shows that, at low viscosity, the flagellar beat of a human sperm cell typically exhibits a considerable chiral component. This follows from the fact that the flagellum never appears as a straight line (in contrast to bull sperms at same viscosity, compare Video 4). Scale bar 20 μm.
Human sperm cells swimming in a high-viscosity fluid (20 mPa·s) near the wall of a planar channel.
The video shows that, at very high viscosity, the chiral beat component becomes considerably weaker for there now exist instances where the flagellum appears as an almost straight line, indicating that the beat pattern approaches the shape of a planar rotating wave. Scale bar 20 μm.
Bull sperm cells swimming in a low-viscosity fluid (3 mPa·s) near the wall of a planar channel.
The video shows that, even at low viscosity, the flagellar beat of bull sperm is approximately planar. This follows from the fact that at certain instances the flagellum appears as a line (in contrast to human sperms at same viscosity, compare Video 2). Scale bar 20 μm.
Reorientation of a human sperm cell swimming in a low-viscosity fluid (1 mPa·s) in a planar channel, after a sudden reversal of the flow direction at time t = 0.
https://doi.org/10.7554/eLife.02403.009Reorientation of two human sperm cells, swimming in a high-viscosity fluid in a planar channel, after a sudden reversal of the flow direction at time t = 0.
https://doi.org/10.7554/eLife.02403.010Additional files
-
Supplementary file 1
This file contains a detailed mathematical derivation of the minimal model in Equations 1 and 2 of the main text, a description of the parameter estimation procedure and a brief summary of numerical methods.
- https://doi.org/10.7554/eLife.02403.013
-
Supplementary file 2
This file contains data tables that summarise the statistical information for each experiment.
- https://doi.org/10.7554/eLife.02403.014





