Kinase Activity: Probing conformational dynamics to understand kinase inhibition
Protein kinases are enzymes that act as molecular switches for signaling pathways in cells. Most kinases have an “on”-state that promotes signaling, and an “off”-state that does not, and these two states have different shapes or conformations. A number of diseases are associated with kinases not functioning properly, so kinases are important targets for researchers working in drug development.
In general, when a protein kinase is switched on, ATP (a molecule that provides cells with energy) binds to the active site of the kinase, and the kinase facilitates the transfer of a phosphate group from ATP to a protein. This means that it is possible to inhibit a kinase by getting a small molecule to bind to the active site instead of ATP. Some of these inhibitors can bind to just one state, but others can bind to both the on-state and the off-state.
To develop better drugs it is important to understand how kinase conformations relate to signaling and how inhibitor molecules select for these conformations. Now, in eLife, Natalie Ahn (University of Colorado, Boulder) and colleagues – including Jake Anderson as first author – report the results of experiments on a kinase called ERK2 that is involved in many signaling pathways in cells (Anderson et al., 2023). ERK2 is of particular interest because it is a key player in the MAPK signaling pathway that is often misregulated in human cancers (Roskoski, 2019; Lavoie et al., 2020).
In many kinases the on-state and the off-state have very different confirmations, but that it not the case for ERK2 (Zhang et al., 1994; Canagarajah et al., 1997). Indeed, the difference is so subtle that it is difficult to distinguish between the two states with X-ray crystallography, a technique that is widely used to determine the static structures of biomolecules. Fortunately, the dynamics of the two states are different, so Anderson et al. were able to use two established techniques for the study of dynamics – nuclear magnetic resonance and hydrogen-deuterium exchange mass spectrometry – to examine the dynamics of ERK2. They found that the the on-state corresponds to at least two conformational substates (named R and L) that interconvert on a timescale of milliseconds.
First, the researchers – who are based the University of Colorado in Boulder, the University of Colorado Anschutz Medical Center and Genentech – tested the impact of three inhibitors on the ERK2 kinase. They found ERK2 is predominantly in the R-state after it binds to an inhibitor called VTX11e, and similarly when it binds to BVD523. However, when ERK2 binds to an inhibitor called GDC0994, it remains in both the R-state and the L-state. What chemical motifs in VTX11e and BVD523 select for the ERK2 R-state? In an efort to answer this question the researchers tested a series of 17 ERK2 inhibitors that were similar to GDC0994 in various ways (see Figure 3 of Anderson et al., 2023). For three of these inhibitors the ERK2 kinase remained in both the R-state and the L-state, but in thirteen cases it selected the R-state (similar to the behavior observed for VTX11e and BVD523.) The remaining inhibitor did not fall neatly into either category.
To better understand the mechanism underlying such selection, Anderson et al. determined the three-dimensional structure of several inhibitors bound to ERK2. They observed that the large bulky chemical group on the right side of GDC0994 may have prevented conformational selection of the R-state, as it did for other inhibitors. The three inhibitors for which the ERK2 kinase remained in both the R-state and the L-state also had similar bulky chemical groups on their right sides, whereas the thirteen inhibitors that selected the R-state did not (Figure 1A). This observation prompted the researchers to ask what would happen if the same bulky group was added to the right side of an inhibitor that would normally select the R-state of ERK2. Luckily, an inhibitor called ATG017 has such a structure, and Anderson et al. found that it was able to bind to both the R-state and the L-state of ERK2, just as GDC0994 does (Figure 1B). This is consistent with previous work on ATG017 (also known as AZD0364) by AstraZeneca (Ward et al., 2019; Flemington et al., 2021).
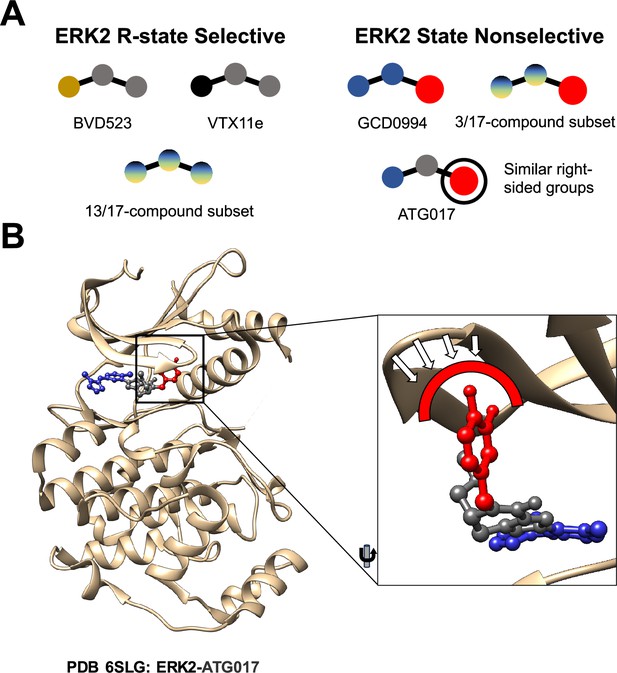
Comparing different ERK2 inhibitors.
(A) BVD523, VTX11e and 13 of the 17 ERK2 inhibitors studied by Anderson et al. select for the R-state when binding to the ERK2 kinase. GCD0994, ATG017 and 3 of the 17 inhibitors do not select for the R-state: these inhibitors have similar structures, with a bulky chemical group (red circle) on their right side. (B) A close-up view of ATG017 (red/grey/blue) bound to ERK2 (beige). With most inhibitors the Gly-loop of ERK2 can bend downwards into a highly stable closed position that is associated with the R-state. With ATG017, however, the bulky chemical group (red) prevents the Gly-loop from reaching this position, so ERK2 is free to assume more than one conformation.
The work of Anderson et al. also sheds light on how the spatial configuration of atoms in ERK2, the inhibitors and even the surrounding solvent contribute to promoting these conformational preferences. For example, when an R-state-selective inhibitor binds to ERK2, movements in the upper lobe of the kinase bring the residues required for catalytic activity closer together. Furthermore, ERK2 is regulated in an allosteric manner (that is, the regulatory site and the active site are different), and Anderson et al. show that the residues involved in allosteric regulation re-arrange their positions in order to relay an allosteric signal from the regulatory site to the active site. Additionally, certain R-state-selective inhibitors influenced the flexibility of the activation loop of ERK2, which prompted Anderson et al. to suggest that these inhibitors might also influence how ERK2 is regulated by other proteins.
In summary, Anderson et al. show how small changes in ERK2 inhibitors might lead to larger changes in ERK2 dynamics. Specifically, their work provides a roadmap for how to engineer and study compounds that preferentially bind the ERK2 kinase in the R-state. Moreover, it will be interesting to explore if comparable phenomena occur with other similar kinases.
References
-
ERK signalling: A master regulator of cell behaviour, life and fateNature Reviews Molecular Cell Biology 21:607–632.https://doi.org/10.1038/s41580-020-0255-7
-
Targeting ERK1/2 protein-serine/threonine kinases in human cancersPharmacological Research 142:151–168.https://doi.org/10.1016/j.phrs.2019.01.039
Article and author information
Author details
Publication history
- Version of Record published: October 18, 2023 (version 1)
Copyright
© 2023, Outhwaite and Seeliger
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 504
- views
-
- 63
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Biochemistry and Chemical Biology
- Microbiology and Infectious Disease
To date, all major modes of monoclonal antibody therapy targeting SARS-CoV-2 have lost significant efficacy against the latest circulating variants. As SARS-CoV-2 omicron sublineages account for over 90% of COVID-19 infections, evasion of immune responses generated by vaccination or exposure to previous variants poses a significant challenge. A compelling new therapeutic strategy against SARS-CoV-2 is that of single-domain antibodies, termed nanobodies, which address certain limitations of monoclonal antibodies. Here, we demonstrate that our high-affinity nanobody repertoire, generated against wild-type SARS-CoV-2 spike protein (Mast et al., 2021), remains effective against variants of concern, including omicron BA.4/BA.5; a subset is predicted to counter resistance in emerging XBB and BQ.1.1 sublineages. Furthermore, we reveal the synergistic potential of nanobody cocktails in neutralizing emerging variants. Our study highlights the power of nanobody technology as a versatile therapeutic and diagnostic tool to combat rapidly evolving infectious diseases such as SARS-CoV-2.
-
- Biochemistry and Chemical Biology
Phosphoinositide 3-kinase (PI3K) beta (PI3Kβ) is functionally unique in the ability to integrate signals derived from receptor tyrosine kinases (RTKs), G-protein coupled receptors, and Rho-family GTPases. The mechanism by which PI3Kβ prioritizes interactions with various membrane-tethered signaling inputs, however, remains unclear. Previous experiments did not determine whether interactions with membrane-tethered proteins primarily control PI3Kβ localization versus directly modulate lipid kinase activity. To address this gap in our knowledge, we established an assay to directly visualize how three distinct protein interactions regulate PI3Kβ when presented to the kinase in a biologically relevant configuration on supported lipid bilayers. Using single molecule Total Internal Reflection Fluorescence (TIRF) Microscopy, we determined the mechanism controlling PI3Kβ membrane localization, prioritization of signaling inputs, and lipid kinase activation. We find that auto-inhibited PI3Kβ prioritizes interactions with RTK-derived tyrosine phosphorylated (pY) peptides before engaging either GβGγ or Rac1(GTP). Although pY peptides strongly localize PI3Kβ to membranes, stimulation of lipid kinase activity is modest. In the presence of either pY/GβGγ or pY/Rac1(GTP), PI3Kβ activity is dramatically enhanced beyond what can be explained by simply increasing membrane localization. Instead, PI3Kβ is synergistically activated by pY/GβGγ and pY/Rac1 (GTP) through a mechanism consistent with allosteric regulation.






