Infectious Diseases: Mathematics and malaria
Parasites are a lot like teenagers. Take a few hundred adolescents in school, each with unique genetic and cultural influences, and you would expect to see huge diversity in behaviour. Instead, we generally find that teenagers fall into one of a small number of groups that have their own distinct identities and patterns of behaviour (or misbehaviour). Similarly, the genetic make-up of Plasmodium falciparum, the parasite that causes most fatal malaria cases, suggests that many different strains of malaria should exist in a host population: in reality, however, only a small number of strains, each with distinctive clinical and epidemiological features, are observed. Peer pressure is the driving force for a finite set of teenager behaviours (Brown et al., 1986), and the immune response of humans appears to be the driving force for the same effect in many pathogens.
Malaria, which arises from P. falciparum carried by mosquitos, is thought to kill more than a million people a year and to infect many more. P. falciparum causes malaria by entering red blood cells and releasing antigens that change the surfaces of the cells; these infected cells then accumulate in the brain and various small blood vessels. The virulence of P. falciparum is believed to arise from its ability to evade the human immune system by changing the antigens that are released. Many important pathogens, including P. falciparum, constantly exchange genetic material: however, the number of strains of malaria (and other diseases) is much lower than expected, given the level of genetic exchange that happens. This has led to ‘strain theory’—the idea that populations of parasites are restricted to specific subtypes (that is, different strains) as a result of their interactions with human immunity (Gupta et al., 1994, 1996). Now, writing in eLife, Yael Artzy-Randrup and co-workers at the University of Michigan, New York University and Princeton University extend strain theory and conclude that immune selection markedly influences populations of P. falciparum, restricting their diversity to a finite number of antigenic strains (Artzy-Randrup et al., 2012).
They focus on the family of 50–60 var genes that code for the PfEMP1 antigens that are central to malarial disease. Each parasite expresses a single var gene at a time, while the rest remain transcriptionally silent. However, as the hosts develop antibodies against the single antigen that is being produced, small sub-populations of P. falciparum begin to produce other types of antigens so as to re-establish the infection. Artzy-Randrup and co-workers developed an ‘agent-based model’ to describe interactions between parasites and humans through simple mathematical rules that quantify genetic and epidemiological processes (Bauer et al., 2009).
The team's model predicts that host immune selection can cause the local P. falciparum population to self-organise into a finite number of strains (see Figure 1), with the degree of genetic overlap between the strains (defined as the number of common var alleles) depending on the transmission intensity (defined as the number of bites per year). The strains do not, in general, have common var alleles, but partially overlapping var alleles can arise when the transmission intensity is high, as it is in many parts of Africa. High transmission intensities also lead to immunity being established in humans at earlier ages than happens in areas with lower transmission intensities, such as parts of Asia: the proportion of people who become immune is also higher in areas with high transmission intensities. The changes in the parasite population appear to be mostly a product of the traits of immune response of humans, while ‘neutral’ factors, such as genetic recombination from random mating, appear to have much less influence.
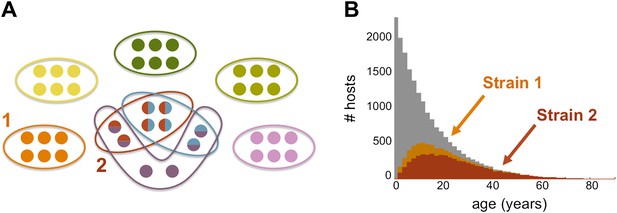
The genome of P. falciparum contains between 50 and 60 var genes that code for different antigens, all of which can lead to malaria. Despite this diversity, only a relatively small number of strains of malaria become established in any population: moreover, these strains tend to have little or no genetic overlap with each other. (A) This illustration (created by Artzy-Randrup et al. and motivated by real data) shows how 40 var genes (circles) can lead to 8 different strains of P. falciparum malaria (each composed of a subset of 6 genes). Five of these strains are unique as they do not genetically overlap with any of the other strains; two strains (shown in red and light blue) share all of their genes with other strains, and one strain (shown in purple) shares four of its genes with other strains. (B) The number of hosts immune to two strains of malaria versus age: strain 1 (orange) is genetically unique, so immunity to this strain is gained only through exposure to it; strain 2 (red) shares genes with other strains of malaria, so immunity to this strain can be acquired through exposure to it and also through exposure to two other strains of malaria. Immunity to strain 1 is higher than for strain 2 as human hosts are exposed to strain 1 at a higher level and at younger ages than strain 2. The grey bars show the host age distribution.
FIGURE: ARTZY-RANDRUP ET AL.
It has long been recognized that malaria infection in humans varies greatly from place to place, and it has been assumed that these differences arise from biological differences in otherwise morphologically similar parasites. As early as 1910, Ronald Ross—the British doctor who won the Nobel Prize in Physiology or Medicine in 1902 for demonstrating that mosquitos transmit the parasites that cause malaria—had noted different types of fever in malaria patients. Then, in work that was just as influential as the work that won him the Nobel Prize, Ross developed mathematical models to describe the spread of malaria (Ross, 1916), which eventually paved the way for the spraying-based strategies that have been widely used in an effort to stop the spread of the disease (see McKenzie et al., 2008 for an historical review).
The biological differences in P. falciparum presumably lead to large variations in its ability to infect mosquitos (only some species of mosquitoes can acquire the parasite), in the patterns of human infection, and in its susceptibility to drugs (Snow et al., 2012). The suggestion by Artzy-Randrup et al. that lower intensity transmission is linked with loss of immunity might be consistent with recent findings from India which suggest that malaria kills substantial numbers of rural adults who lack access to curative therapies (Dhingra et al., 2010).
Mosquito nets treated with insecticides, spraying insecticides inside houses and, most importantly, drugs (notably artemisinin-combination therapies) have proved to be powerful tools in the fight against malaria (Breman et al., 2006). Strain theory suggests that vaccines should be possible, but an effective malaria vaccine has yet to be found: indeed, a recent trial of the RTS,S/AS01 vaccine found that it provided only ‘modest protection against both clinical and severe malaria in young infants’ (The RTS,S clinical trials partnership, 2012).
Malaria is increasingly the subject of attention around the world, and a better understanding of the basic biologic processes involved will be central to developing the tools which can better control this lethal, but curable disease. More than a century after the pioneering work of Ross, malaria still presents a formidable challenge to medical science.
References
-
Agent-based modeling of host–pathogen systems: the successes and challengesInf Sci (Ny) 179:1379–1389.
-
BookChapter 21: Conquering malariaIn: Jamison DT, Breman JG, Measham AR, Alleyne G, Claeson M, Evans DB, Jha P, Mills A, Musgrove P, editors. Disease control priorities in developing countries (2nd Ed). Washington, DC: World Bank. pp. 413–432.
Article and author information
Author details
Publication history
- Version of Record published: December 18, 2012 (version 1)
Copyright
© 2012, Jha
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 720
- views
-
- 59
- downloads
-
- 1
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Microbiology and Infectious Disease
In the Firmicutes phylum, GpsB is a membrane associated protein that coordinates peptidoglycan synthesis with cell growth and division. Although GpsB has been studied in several bacteria, the structure, function, and interactome of Staphylococcus aureus GpsB is largely uncharacterized. To address this knowledge gap, we solved the crystal structure of the N-terminal domain of S. aureus GpsB, which adopts an atypical, asymmetric dimer, and demonstrates major conformational flexibility that can be mapped to a hinge region formed by a three-residue insertion exclusive to Staphylococci. When this three-residue insertion is excised, its thermal stability increases, and the mutant no longer produces a previously reported lethal phenotype when overexpressed in Bacillus subtilis. In S. aureus, we show that these hinge mutants are less functional and speculate that the conformational flexibility imparted by the hinge region may serve as a dynamic switch to fine-tune the function of the GpsB complex and/or to promote interaction with its various partners. Furthermore, we provide the first biochemical, biophysical, and crystallographic evidence that the N-terminal domain of GpsB binds not only PBP4, but also FtsZ, through a conserved recognition motif located on their C-termini, thus coupling peptidoglycan synthesis to cell division. Taken together, the unique structure of S. aureus GpsB and its direct interaction with FtsZ/PBP4 provide deeper insight into the central role of GpsB in S. aureus cell division.
-
- Microbiology and Infectious Disease
The agr quorum-sensing system links Staphylococcus aureus metabolism to virulence, in part by increasing bacterial survival during exposure to lethal concentrations of H2O2, a crucial host defense against S. aureus. We now report that protection by agr surprisingly extends beyond post-exponential growth to the exit from stationary phase when the agr system is no longer turned on. Thus, agr can be considered a constitutive protective factor. Deletion of agr resulted in decreased ATP levels and growth, despite increased rates of respiration or fermentation at appropriate oxygen tensions, suggesting that Δagr cells undergo a shift towards a hyperactive metabolic state in response to diminished metabolic efficiency. As expected from increased respiratory gene expression, reactive oxygen species (ROS) accumulated more in the agr mutant than in wild-type cells, thereby explaining elevated susceptibility of Δagr strains to lethal H2O2 doses. Increased survival of wild-type agr cells during H2O2 exposure required sodA, which detoxifies superoxide. Additionally, pretreatment of S. aureus with respiration-reducing menadione protected Δagr cells from killing by H2O2. Thus, genetic deletion and pharmacologic experiments indicate that agr helps control endogenous ROS, thereby providing resilience against exogenous ROS. The long-lived ‘memory’ of agr-mediated protection, which is uncoupled from agr activation kinetics, increased hematogenous dissemination to certain tissues during sepsis in ROS-producing, wild-type mice but not ROS-deficient (Cybb−/−) mice. These results demonstrate the importance of protection that anticipates impending ROS-mediated immune attack. The ubiquity of quorum sensing suggests that it protects many bacterial species from oxidative damage.





