miR-124 controls male reproductive success in Drosophila
Decision letter
-
Mani RamaswamiReviewing Editor; Trinity College, Dublin, Ireland
eLife posts the editorial decision letter and author response on a selection of the published articles (subject to the approval of the authors). An edited version of the letter sent to the authors after peer review is shown, indicating the substantive concerns or comments; minor concerns are not usually shown. Reviewers have the opportunity to discuss the decision before the letter is sent (see review process). Similarly, the author response typically shows only responses to the major concerns raised by the reviewers.
Thank you for sending your work entitled “miR-124 controls male reproductive success in Drosophila” for consideration at eLife. Your article has been favorably evaluated by a Senior editor and 3 reviewers, of whom, Mani Ramaswami, is a member of our Board of Reviewing Editors.
The Reviewing editor and the other reviewers discussed their comments before we reached this decision, and the Reviewing editor has assembled the following comments to help you prepare a revised submission.
This manuscript describes male-specific behavioral defects in miR-124 mutants, nicely demonstrating that miR-124 is required for the development/expression of male-specific courtship and aggressive behavior in Drosophila. These behavioral defects of miR-124 mutants, as well as associated changes in production of the pheromone cVA, are linked to increased expression of transformer (tra), a splicing factor that regulates the male-specific splicing of its downstream targets, dsx and fru. Several lines of evidence show tra to be a target of miR-124, and reduction of tra is shown to suppress miR-124 mutant phenotypes. As cVA is a cuticular hydrocarbon of males flies that mediates male–female attraction, as well as male–male repulsion, several of the behavioral defects of miR-124 are proposed to be explained by the dysregulation of one downstream target, tra, and its role in the production of sex-specific pheromones. Together, this supports a model in which endogenous miR-124 (and therefore potentially other miRNAs) act to suppress phenotypes that may result from the leaky regulation of tissue and sex-specific mRNA splicing.
In terms of novelty and interest, this is appropriate for publication in eLife. However, several additional experiments and substantive revisions are required to strengthen some key conclusions. The most important of which are essential to convincingly explain the links between altered tra expression, altered pheromone production, and behavior.
1) As tra function in sex determination is mediated through its effects on fru and dsx, and a major hypothesis here is that increased levels of tra would result in leaky regulation of sex-specific splicing, it would be valuable to assess how the expression of these transcripts downstream of tra is affected miR-124 mutants. Leaky traF would suggest the male makes less Fru, less DsxM, and some DsxF.
2) It is necessary to establish the cell type in which miR-124 repression of tra occurs, as relevant to the production of cVA. Is miR-124 expressed in oenocytes? Is reduction of tra in miR-124 oenocytes sufficient to suppress miR-124 mutant phenotypes? Is reduction of tra in the nervous system sufficient (if so, then this should be explained), or not, to suppress miR-124 mutant phenotypes?
3) An alternative hypothesis for miR-124 behavioral phenotypes is that increased levels of tra in miR-124 mutants results in altered development/morphology of sex-specific neural circuitry. An analysis of the anatomy of fru or dsx expressing neurons in brains of miR-124 mutant and miR-124 mutant flies with reduced tra levels will allow a deeper understanding of the link between elevated tra expression in miR-124 mutants and associated behavioral phenotypes.
4) Throughout the text and figure legends the authors should be explicit about which genotypes have been used, particularly for miR-124 mutant males. Figure 1A, what is the deficiency used? Figure 1B, what is the miR-124 mutant male genotype? Complete genotypes are also needed in Figure 2, Figure 4, and Figure 6D.
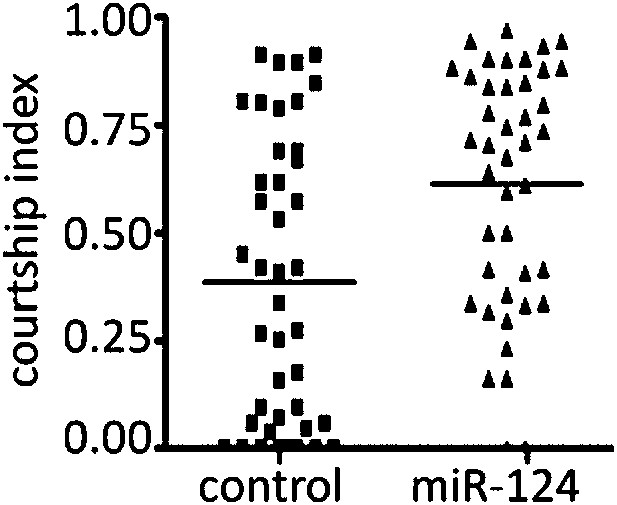
5) Figure 1D, the CI for the control flies is unusually low: what are the reasons for this? Male–female courtship values seem to show an unacceptably large distribution. It is not clear from these data (Figure 1) whether or not miR-124 affects male–female courtship.
6) The origins, details, and properties of the miR-124 mutant, rescuing transgene, sponge and miR-124-Gal4 should be provided in the Materials and methods.
https://doi.org/10.7554/eLife.00640.018Author response
1) As tra function in sex determination is mediated through its effects on fru and dsx, and a major hypothesis here is that increased levels of tra would result in leaky regulation of sex-specific splicing, it would be valuable to assess how the expression of these transcripts downstream of tra is affected miR-124 mutants. Leaky traF would suggest the male makes less Fru, less DsxM, and some DsxF.
Less dsxM transcript was detected in head samples from miR-124 adult males. However, we did not observe an increase of dsxF transcript. This is somewhat unexpected. The conventional model is that tra should switch splicing between the dsxM and dsxF forms. It is possible that production of the two Dsx splice products does not vary linearly with traF levels. We speculate on a possible molecular explanation in the revised Discussion (with data provided in Figure 8).
We were unable to measure fruM levels by qPCR. None of the 6 pairs of primers tested gave a specific PCR product (multiple products formed).
2) It is necessary to establish the cell type in which miR-124 repression of tra occurs, as relevant to the production of cVA. Is miR-124 expressed in oenocytes? Is reduction of tra in miR-124 oenocytes sufficient to suppress miR-124 mutant phenotypes? Is reduction of tra in the nervous system sufficient (if so, then this should be explained), or not, to suppress miR-124 mutant phenotypes?
miR-124 is not expressed in oenocytes.
Removing oenocytes does not affect cVA production (Billeter et al 2009). It is unlikely that the miRNA is acting in oenocytes, so we have not tested the effects of reducing tra in oenocytes in the miR-124 mutant.
Several lines of evidence indicate that miR-124 acts in the nervous system:
(A) Reduction of miR-124 activity in neurons is sufficient to reproduce the male–male courtship phenotype. miR-124 was depleted in neurons by expressing the miR-124 sponge using elav-Gal4. Data are shown in the new Figure 5C.
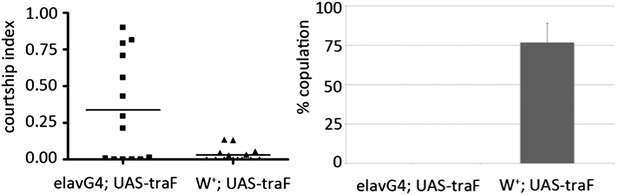
(B) elav-Gal4 driven expression of TraF increased male–male courtship and reduced completion of male–female courtship (Author response image 1). Elevated TraF in the CNS is sufficient to reproduce the effects of the miR-124 mutant.
(C) Selective depletion of tra mRNA in the CNS offset the male–male courtship phenotype in the miR-124 mutant background. The level of male–male courtship was comparable in the elav-Gal4>UAStraRNAi control compared to the elav-Gal4>UAStraRNAi in the miR-124 mutant. Data are shown in the new Figure 6F.
3) An alternative hypothesis for miR-124 behavioral phenotypes is that increased levels of tra in miR-124 mutants results in altered development/morphology of sex-specific neural circuitry. An analysis of the anatomy of fru or dsx expressing neurons in brains of miR-124 mutant and miR-124 mutant flies with reduced tra levels will allow a deeper understanding of the link between elevated tra expression in miR-124 mutants and associated behavioral phenotypes.
This hypothesis would lead to the expectation of a change in the behaviour of the miR-124 mutant male. The data on courtship behaviour do not support this view.
First, there was no difference in the behaviour of the miR-124 mutant male toward control CS males in the male courtship assay (Figure 2A). Second, interaction with behaviorally inert (decapitated) mutant males elicited behavioral changes in wild-type males. These observations cannot be explained by changes in the neural circuitry in the mutant male brain. We suggest that the pheromone experiments provide a more likely explanation for the phenotypes.
We have looked at FruM expression in miR-124 mutant and control brains (fruP1-gal4>CD8RFP and anti FruM). We saw no obvious differences. For the reasons above, we do not think it would be fruitful to look in depth for subtle changes.
4) Throughout the text and figure legends the authors should be explicit about which genotypes have been used, particularly for miR-124 mutant males. Figure 1A, what is the deficiency used? Figure 1B, what is the miR-124 mutant male genotype? Complete genotypes are also needed in Figure 2, Figure 4, and Figure 6D.
We intended to provide full genotype information in the Materials and methods section, but we now see that there were a few details missing. The text has been revised to provide complete information, as requested.
5) Figure 1D, the CI for the control flies is unusually low: what are the reasons for this? Male–female courtship values seem to show an unacceptably large distribution. It is not clear from these data (Figure 1) whether or not miR-124 affects male–female courtship.
The point of this experiment was to ask whether the reason for the low mating success shown in Figure 1A was due reduced courtship activity by mutant males.
Figure 1A shows that mating success was reduced for the mutant males. Figures 1B–D explore the stage at which the mutant males fail. B and C exclude early stage effects. Figure 1D was designed to ask if the defect in 1A was due to reduced courtship activity by the mutant males. For this we used decapitated female targets, to remove female behavioural input. The mutants did not seem to show reduced CI compared to the CS control when tested with behaviorally inert females.
The reviewers are correct to note that the variance is large in this data set. This is true for both the control and the mutant. We think it may be helpful to present the data as a scatter plot to give a better feel for the variance (Author response image 2).
The data is borderline significant at p<0.05 for the difference between the median of 56 pairs of flies. (The original Figure 1D showed the data comparing the means of 4 sets of 14 pairs. Analysed that way the difference was borderline not significant.)
For these experiments the mutant was backcrossed for 6 generations into the CS control background. The two populations should be very similar, except at the miR-124 locus. The flies used for the experiments in Figure 1A–D were from the same amplified populations. We do not have an explanation for the variance and low CI, but note that whatever causes this in the control should also do so in the mutant (whether the cause is genetic or environmental in origin, we have controlled the two populations as best we can).
That said, the conclusion we draw from this experiment is that there is no evidence for reduced courtship activity by the mutant male. We are not trying to use this data as evidence in support of a behavioural difference. If the editors prefer, we are willing to remove this experiment. The result is not essential to the logical flow of the manuscript.
6) The origins, details, and properties of the miR-124 mutant, rescuing transgene, sponge and miR-124-Gal4 should be provided in the Materials and methods.
Done.
https://doi.org/10.7554/eLife.00640.019