Cancer: A new role for an old enemy
Many human cancers rely on the abnormal activation of a protein called AKT to promote the growth and survival of tumors. AKT, which is also known as protein kinase B, is therefore a potential target for anti-cancer therapies. Several compounds that inhibit the kinase activity of AKT have been identified, but progressing these into the clinic requires a more complete understanding of their effect on AKT in cancer cells (Fang et al., 2013; Dienstmann et al., 2014).
Now, in eLife, Ingo Mellinghoff of the Memorial Sloan-Kettering Cancer Center (MSKCC) and colleagues—including Igor Vivanco and Zhi Chen as joint first authors—address this issue by examining how two different types of AKT inhibitor regulate the survival of cancer cells. This revealed a previously unrecognized role for AKT that is independent of its role as a kinase (Vivanco et al., 2014).
AKT is a component of a signaling network within cells that responds to signals that promote cell growth and survival. It is activated by another protein called PI3K that generates specific lipid molecules to recruit AKT to the cell membrane (Figure 1). In human cancers this signaling network is frequently disturbed by mutations that cause PI3K and/or AKT to be more active (Fruman and Rommel, 2014).
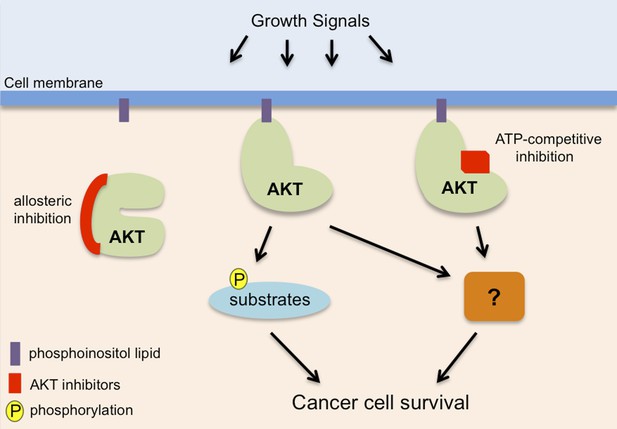
AKT, also known as protein kinase B, promotes cancer cell survival in two distinct ways.
AKT (pale green) is recruited to phosphoinositol lipids (purple) at the cell membrane. Normally it is only activated in response to growth or survival signals, but it has increased activity in many cancers. It has been known for some time that AKT promotes the survival of cancer cells by adding phosphate groups (yellow) to protein substrates (light blue): this process involves ATP (not shown) binding to an active site in the kinase domain of the AKT, so it can be inhibited by drugs that compete with ATP to bind to this site (red rectangle). Vivanco, Chen et al. show that AKT can also promote cancer cell survival in a way that is independent of its kinase function: however, the details of this process remain unclear (hence the question mark). Drugs that compete with ATP do not inhibit this kinase-independent role, but allosteric inhibitors (left; see main text) inhibit both the kinase-dependent and kinase-independent roles of AKT, so they have the potential to be more effective therapies to treat cancer.
Some inhibitors work by binding to the active site of the enzyme domain within the AKT protein: this prevents ATP binding to the active site and thus inhibits the kinase activity of AKT. Unfortunately the clinical use of inhibitors that work by competing with ATP binding has been limited due to their toxic side effects, which may be due to them also targeting other kinases. Other inhibitors work by altering the shape or ‘conformation’ of AKT in a process known as allosteric inhibition (Fang et al., 2013; Dienstmann et al., 2014). An allosteric inhibitor that works by stabilizing AKT in an inactive state—which prevents ATP or other proteins binding to the active site—is currently in Phase II clinical trials.
The study by Vivanco, Chen et al.—who are based at MSKCC, the Spanish National Cancer Research Center and Cornell University—found that this allosteric inhibitor was better at killing cancer cells than inhibitors that compete with ATP binding. This suggested that AKT has an additional role in the survival of cancer cells that is independent of its kinase activity. Further support for this idea came from experiments demonstrating that a mutant of AKT with no enzyme activity could protect cancer cells from death.
Although the details of this second role are not clear, Vivanco, Chen et al. show that another domain in AKT called the PH domain is important. PH domains in other proteins generally bind to lipid molecules, but these latest results suggest that the PH domain in AKT may promote cancer cell survival independently of its ability to bind to lipids. Identifying molecules that can bind to kinase-inactive AKT mutants would help to resolve this issue.
Is the kinase-independent role of AKT in promoting cell survival a feature of human cancers? Vivanco, Chen et al. characterized a mutant of AKT with no kinase activity that was originally found in a sample of skin cancer cells. When this mutant version was expressed in skin cells it promoted their survival. Furthermore, they found that the most common mutation in AKT present in cancer cells—which promotes the interaction of AKT with the cell membrane—also promotes cell survival independent of kinase activity. These findings suggest that the kinase-independent role of AKT could have widespread importance in tumor formation.
Other examples of protein kinases that have enzyme independent roles in promoting tumor formation have been reported (e.g., Gustafson et al., 2014; Holderfield et al., 2014; Tan et al., 2015), suggesting that this is a common characteristic. It is predicted that up to 10% of protein kinases found in humans lack kinase activity. These ‘pseudokinases’ are likely to have similar roles to the kinase-independent roles of true protein kinases (Zhang et al., 2012).
The study by Vivanco, Chen et al. highlights the importance of fully appreciating the non-enzymatic roles of proteins in both healthy and diseased cells. It suggests that allosteric inhibitors of AKT are likely to be more useful for treating AKT-dependent cancers than those that specifically target the kinase domain. More generally, it suggests that finding allosteric inhibitors of other protein kinases linked with human diseases may prove to be a fruitful approach to use to develop new therapies.
References
-
Picking the point of inhibition: a comparative review of PI3K/AKT/mTOR pathway inhibitorsMolecular Cancer Therapeutics 13:1021–1031.https://doi.org/10.1158/1535-7163.MCT-13-0639
-
PI3K and cancer: lessons, challenges and opportunitiesNature Reviews Drug Discovery 13:140–156.https://doi.org/10.1038/nrd4204
-
Targeting RAF kinases for cancer therapy: BRAF-mutated melanoma and beyondNature Reviews Cancer 14:455–467.https://doi.org/10.1038/nrc3760
-
The role of pseudokinases in cancerCellular Signalling 24:1173–1184.https://doi.org/10.1016/j.cellsig.2012.01.017
Article and author information
Author details
Publication history
- Version of Record published: February 10, 2015 (version 1)
Copyright
© 2015, Monaghan and Whitmarsh
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,135
- views
-
- 69
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cell Biology
Here, we investigated the mechanisms by which aging-related reductions of the levels of Numb in skeletal muscle fibers contribute to loss of muscle strength and power, two critical features of sarcopenia. Numb is an adaptor protein best known for its critical roles in development, including asymmetric cell division, cell-type specification, and termination of intracellular signaling. Numb expression is reduced in old humans and mice. We previously showed that, in mouse skeletal muscle fibers, Numb is localized to sarcomeres where it is concentrated near triads; conditional inactivation of Numb and a closely related protein Numb-like (Numbl) in mouse myofibers caused weakness, disorganization of sarcomeres, and smaller mitochondria with impaired function. Here, we found that a single knockout of Numb in myofibers causes reduction in tetanic force comparable to a double Numb, Numbl knockout. We found by proteomics analysis of protein complexes isolated from C2C12 myotubes by immunoprecipitation using antibodies against Numb that Septin 7 is a potential Numb-binding partner. Septin 7 is a member of the family of GTP-binding proteins that organize into filaments, sheets, and rings, and is considered part of the cytoskeleton. Immunofluorescence evaluation revealed a partial overlap of staining for Numb and Septin 7 in myofibers. Conditional, inducible knockouts of Numb led to disorganization of Septin 7 staining in myofibers. These findings indicate that Septin 7 is a Numb-binding partner and suggest that interactions between Numb and Septin 7 are critical for structural organization of the sarcomere and muscle contractile function.
-
- Cell Biology
Elastic cartilage constitutes a major component of the external ear, which functions to guide sound to the middle and inner ears. Defects in auricle development cause congenital microtia, which affects hearing and appearance in patients. Mutations in several genes have been implicated in microtia development, yet, the pathogenesis of this disorder remains incompletely understood. Here, we show that Prrx1 genetically marks auricular chondrocytes in adult mice. Interestingly, BMP-Smad1/5/9 signaling in chondrocytes is increasingly activated from the proximal to distal segments of the ear, which is associated with a decrease in chondrocyte regenerative activity. Ablation of Bmpr1a in auricular chondrocytes led to chondrocyte atrophy and microtia development at the distal part. Transcriptome analysis revealed that Bmpr1a deficiency caused a switch from the chondrogenic program to the osteogenic program, accompanied by enhanced protein kinase A activation, likely through increased expression of Adcy5/8. Inhibition of PKA blocked chondrocyte-to-osteoblast transformation and microtia development. Moreover, analysis of single-cell RNA-seq of human microtia samples uncovered enriched gene expression in the PKA pathway and chondrocyte-to-osteoblast transformation process. These findings suggest that auricle cartilage is actively maintained by BMP signaling, which maintains chondrocyte identity by suppressing osteogenic differentiation.





