Pentagone internalises glypicans to fine-tune multiple signalling pathways
Figures
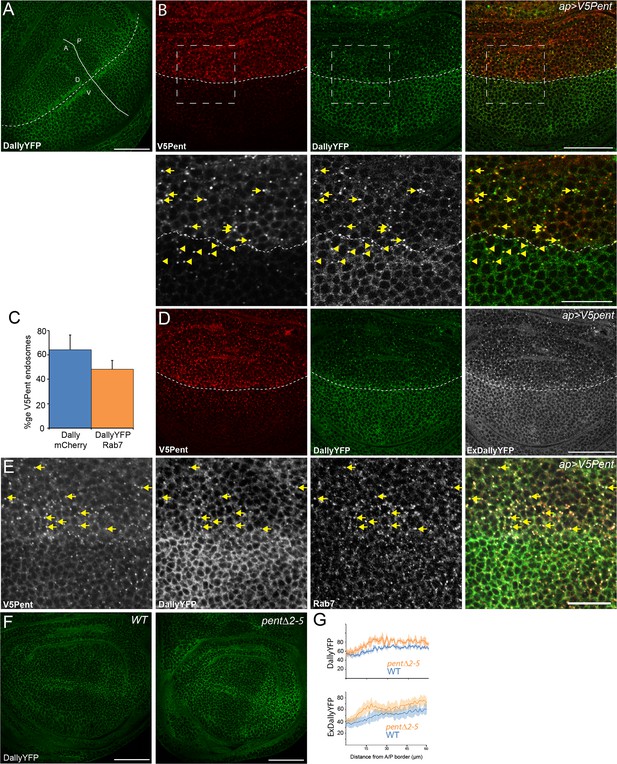
Pent internalises Dally.
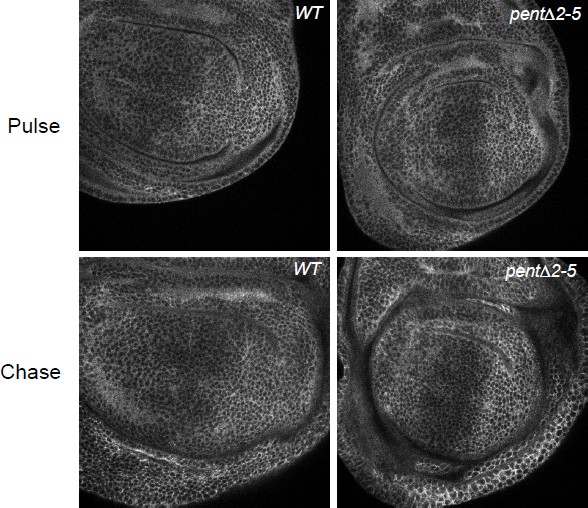
(A) Localisation of DallyYFP in a WT third instar wing disc. Solid and dashed lines indicate the AP and DV boundaries respectively. (B) Expression of UASV5Pent with apGal4 internalised DallyYFP into puncta which co-localise with V5Pent. Arrows and arrowheads show co-localisation in the dorsal compartment and ventral compartments respectively. Dashed line shows DV boundary. (C) Quantification of V5Pent co-localisation with a functional DallymCherry fusion protein (for details on this line see Materials and methods and Figure 1—figure supplement 3A, D, A', D', I) and Rab7YFP in discs expressing UASV5Pent driven by apGal4. 66% of V5Pent endosomes contained Dally, and 49% contained Dally and Rab7. Error bar represents standard deviation (n=10). (D) Expression of UASV5Pent with apgal4 reduces extracellular DallyYFP (grey, right panel) as well as total DallyYFP (green, left). (E) DallyYFP and V5Pent co-localise with the late endosomal marker Rab7. Disc expressing UASV5Pent with apGal4. Endosomes positive for DallyYFP, V5Pent and Rab7 are marked with arrows. (F, G) DallyYFP protein level is increased in pent mutant wing discs. Images show third instar wing discs with DallyYFP autofluorescence in WT and pent△2–5. The graph shows mean data of quantification of fluorescence intensity and extracellular labelling of DallyYFP in the posterior compartment (n=30 in three independent experiments, error bars show standard deviation). Scale bars are 50 µm in upper images and 20 µm in zooms and (E). See also Figure 1—figure supplements 1–4.
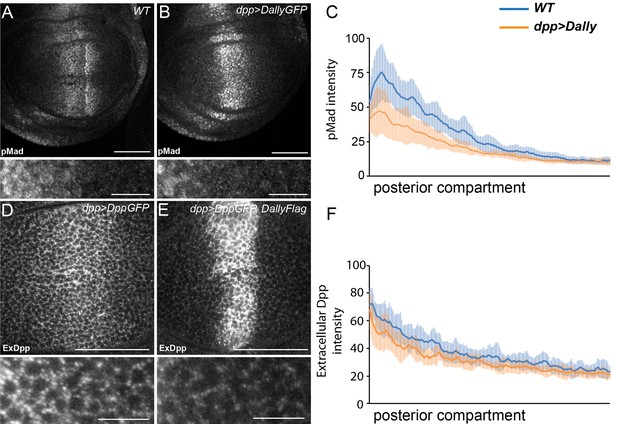
Excessive Dally traps Dpp.
(A–C) WT (A) and disc expressing DallyGFP in the Dpp stripe (B) immunostained for pMad. Expression of Dally in the Dpp stripe increases pMad in the centre of the disc at the expense of the long-range gradient. Fluorescence intensity was measured in the posterior compartment to examine only the non-cell autonomous impact on the width of the pMad gradient, and is quantified in (C), where the blue line is WT and orange line is dppGal4>DallyGFP. Error bars show standard deviation (n=8). (D–F) Third instar discs expressing DppGFP (D) or DppGFP and DallyFlag (E) under control of dppGal4, immunostained for extracellular GFP. Expression of Dally greatly increases extracellular Dpp in the centre, but prevents spreading of Dpp away from the source. Data in the posterior compartment are quantified in (F), where the blue line is DppGFP alone and orange DppGFP and DallyFlag. Error bars show standard deviation (n=9). Scale bars are 50 µm in main images and 10 µm in enlargements.
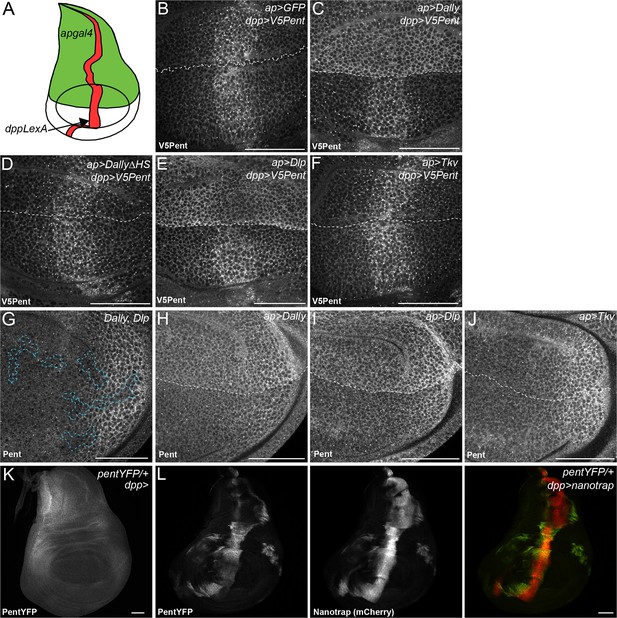
Binding interactions and distribution of Pent.
(A) Schematic diagram of wing disc, where UAS constructs are expressed with apgal4 (green) and LexOV5Pent from the dpp stripe (dppLexA). (B–F) Third instar wing discs expressing UASGFP (B), UASDallyGFP (C), UASDally△HSGFP (D), UASDlpGFP (E) or UASTkvGFP (F) with apGal4, immuno-stained for V5Pent which is expressed in the Dpp stripe. V5Pent accumulates on cells expressing Dally or Dlp. (G) Third instar wing disc with clones mutant for Dally and Dlp (marked by dashed line), stained for Pent. Endogenous Pent does not accumulate in the mutant clones. (H–J) Third instar wing discs expressing UASDallyGFP (H), UASDlpGFP (I) or UASTkvGFP (J) with apGal4 immunostained for Pent. Pent accumulates on cells expressing Dally or Dlp, but not Tkv. (K–L) Control PentYFP wing disc (K) and disc expressing VHH-GFP4::CD8::mCherry, a chimeric membrane protein comprising an extracellular, single domain anti-GFP nanobody and cytoplasmic mCherry under control of dppGal4 (L). In control discs, PentYFP is mostly found in the lateral region of the disc, whereas Pent is seen at high levels in the centre of the disc when the nanotrap is expressed, demonstrating that Pent spreads far from the lateral regions of the disc. Scale bars are 50 µm in all images.
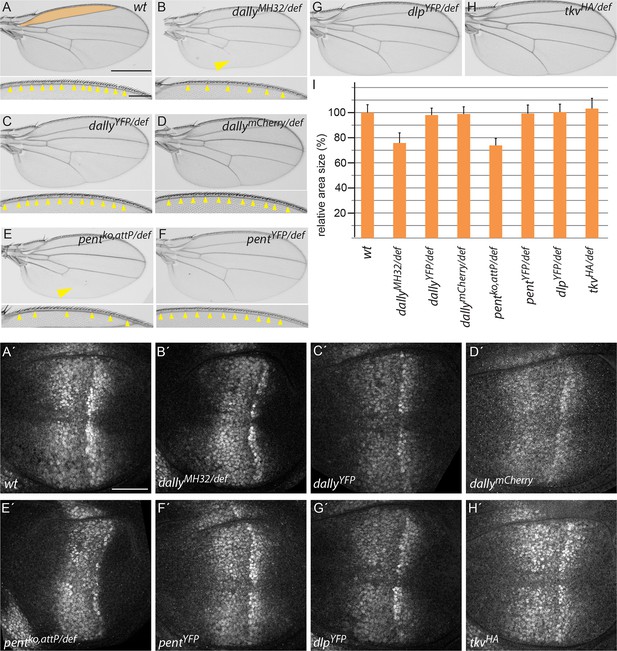
Endogenously tagged BMP signalling components are fully functional.
(A–H) Adult wing and anterior wing margin morphology (for A–F) from flies of the indicated genotypes. Scale bars are shown in (A) and are 500 µm and 100 µm for wing and wing margin, respectively. A size comparison of the anterior lateral regions of the wings (region bordered by longitudinal veins L1 and L2; indicated in orange in the WT (A) wing) is shown in (I). For the quantification, wings of 20–30 male flies were used. Error bars represent standard deviation of the mean. (A’–H’) pMad immunostaining of 3rd instar larvae of the indicated genotypes. Scale bar, 50 µm. Wing venation (yellow arrowhead), margin and lateral size defects of wings lacking dally (B) are fully restored by the dallyYFP (C) or dallymCherry (D) alleles. Similarly, larval discs homozygous for either DallyYFP (C’) or dallymCherry (D’) display a normal pMad pattern as opposed to the weak and constricted pMad distribution of dally mutants (B’). Loss of L5 (arrowhead), reduction of sensory bristles and lateral size deficits (E,I) as well as the compacted pMad distribution of pent mutants (E’) are fully restored in pentYFP (F, F’ and I). Flies with dlpYFP or tkvHA alleles as a sole source for Dlp and Tkv are viable and fertile with completely normal wings (G, H and I). pMad patterns of larval wings homozygous for either allele are indistinguishable from the wild-type pattern (compare G’, H’ to A’).
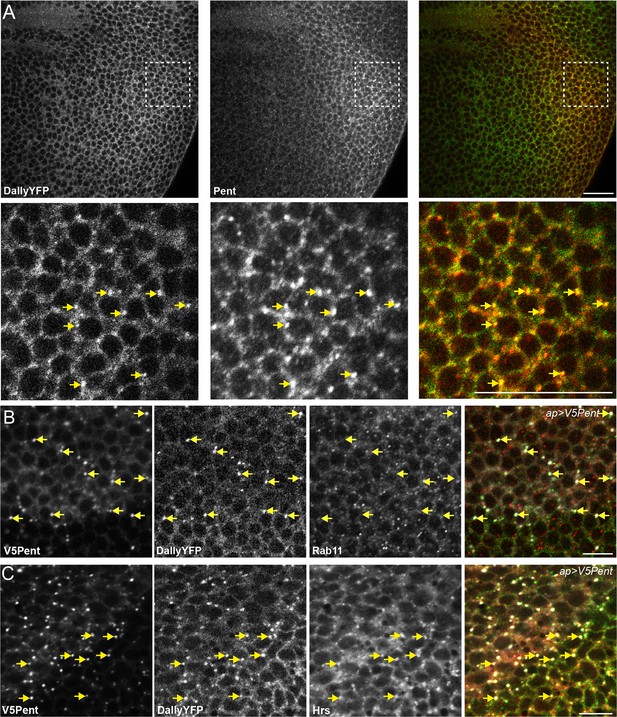
Pent and DallyYFP co-localise at endogenous levels.
(A) Third instar DallyYFP wing disc immunostained for Pent. Pent and DallyYFP localise together in discrete puncta. (B,C) Third instar wing discs expressing UASV5Pent with apGal4. Immuno-staining shows co-localisation of V5Pent-DallyYFP endosomes with Hrs (B) but not Rab11 (C). Arrows show location of dots of V5Pent. Scale bars are 10 µm in (A) and 20 µm in (B, C).
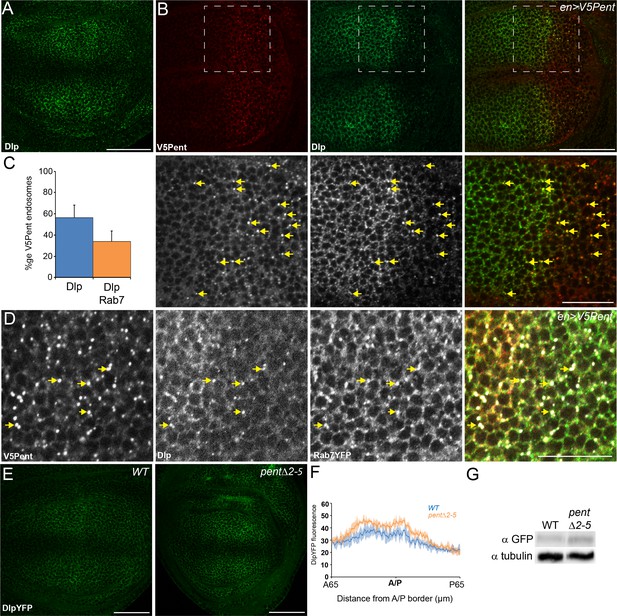
Pent internalises the second D. melanogaster glypican, Dally-like protein.
(A) Localisation of Dlp in a WT third instar wing disc. (B) Expression of UASV5Pent with enGal4 resulted in a decrease of Dlp protein in the posterior compartment and co-localisation of Dlp with V5Pent. Arrows show endosomes where Dlp and V5Pent co-localise. (C) Quantification of V5Pent co-localisation with Dlp and Rab7YFP in discs expressing UASV5Pent driven by apGal4. 57% of V5Pent endosomes contained Dlp, and 34% contained Dlp and Rab7. Error bar represents standard deviation (n=10). (D) Dlp and V5Pent co-localise with the late endosomal marker Rab7. Disc expressing UASV5Pent with enGal4, in a Rab7YFP background. Endosomes positive for Dlp, V5Pent and Rab7 are marked with arrows. (E, F) DlpYFP protein level is increased in pent mutant wing discs. Images show third instar wing discs with DlpYFP autofluorescence in WT and pent△2–5. The graph shows mean data of quantification of fluorescence intensity (n=20 in three independent experiments, error bars show standard deviation). (G) Western blot showing an increase in DlpYFP in pent△2–5 mutant discs compated to WT. Boxes are enlarged in the lower panels. Scale bars are 50 µm, except lower panels of (B) where they are 20 µm. See also Figure 2—figure supplement 1–3.
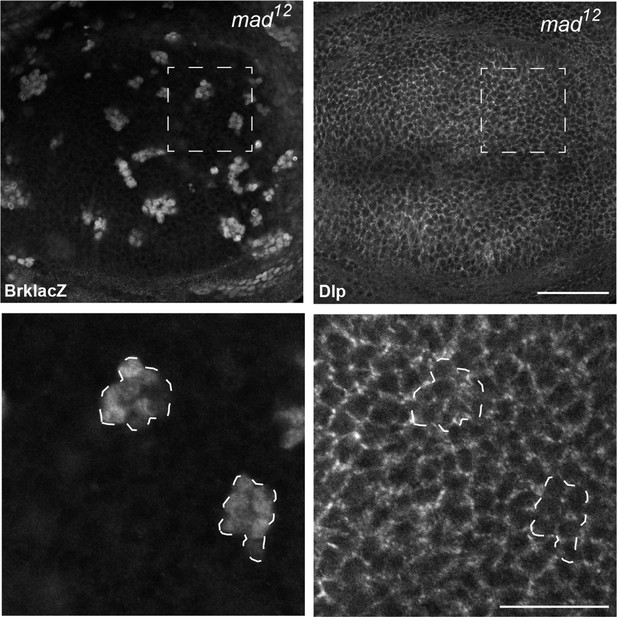
Dlp expression is not repressed by Dpp signalling.
Inhibition of Dpp signalling in Mad mutant clones up-regulates the Dpp repressed gene brk, but does not increase Dlp protein level, suggesting that Dlp is not repressed by Dpp signalling. Scale bars are 50 µm in upper images and 20 µm in enlargements.
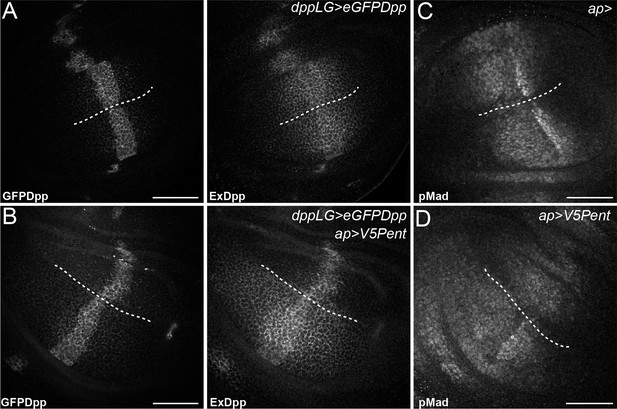
Pent in the dorsal compartment reduces Dpp accumulation and affects pMad gradient formation.
(A–B) Third instar wing disc expressing LexOeGFPDpp under control of DppLG, immunostained for extracellular GFP to visualise spreading of Dpp. Expression of V5Pent in the dorsal compartment with apGal4 (B) reduces extracellular Dpp levels in the dorsal compartment. (C–D) Third instar control wing disc (C) or disc expressing V5Pent under control of apGal4 (D) immunostained for pMad. Expression of Pent reduces and shrinks pMad in the dorsal compartment. Scale bars are 50 mm, the DV compartment boundary is indicated by the dotted line.
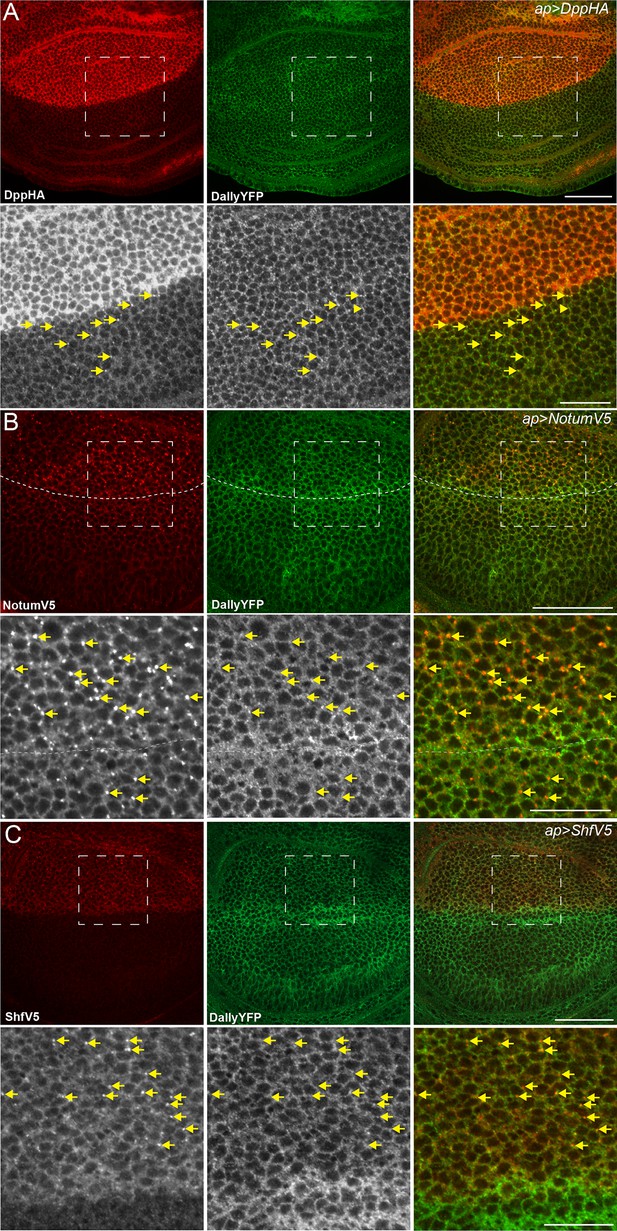
Dpp, Notum and Shf do not internalise DallyYFP.
(A–C) Third instar wing discs expressing UASDppHA (A), UASNotumV5 (B) or UASShfV5 (C) with apGal4. No reduction in DallyYFP is seen in any condition. Lower panels are enlargements of indicated regions in upper panels. Dashed line indicates the DV boundary in (B). Scale bars are 50 µm in upper images and 20 µm in enlarged regions.
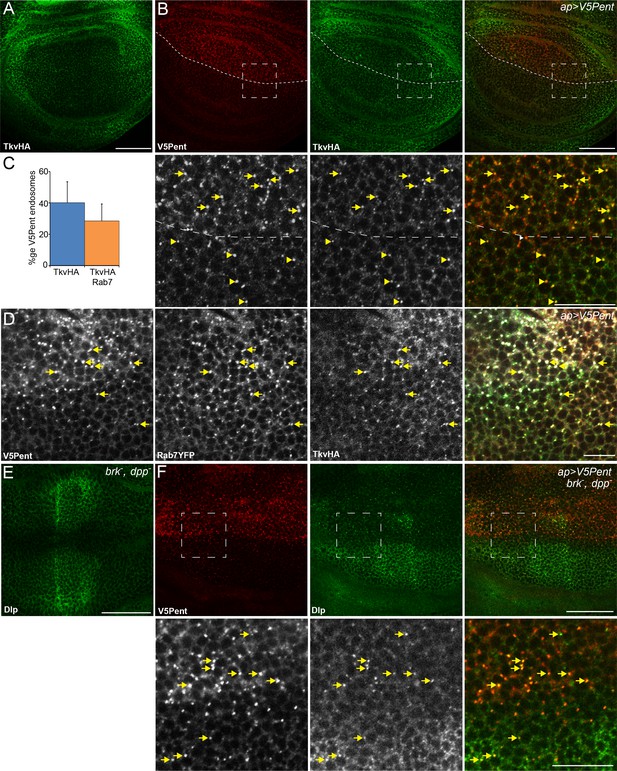
Internalisation of glypicans by Pent is signalling independent.
(A) Localisation of TkvHA in a WT third instar wing disc. (B) UASV5Pent expressed with apGal4 co-localised with TkvHA in puncta, but no reduction in TkvHA protein level was seen. Arrows show co-localisation of Pent and TkvHA in the dorsal compartment, arrowheads in the ventral compartment. (C) Quantification of V5Pent co-localisation with TkvHA and Rab7YFP in discs expressing UASV5Pent driven by apGal4. 40% of V5Pent endosomes contained TkvHA, and 29% contained TkvHA and Rab7. Error bar represents standard deviation (n=10). (D) TkvHA and V5Pent co-localise with the late endosomal marker Rab7. Disc expressing UASV5Pent with apGal4. Endosomes positive for TkvHA, V5Pent and Rab7 are marked with arrows. (E) Localisation of Dlp in a brkXA; dppd12/dppd14 third instar wing disc. There is an increase in intensity in the centre of the disc. (F) Internalisation of Dlp by Pent does not require Dpp. Wing disc expressing UASV5Pent with apgal4 in a dpp mutant background. Arrows show co-localisation of V5Pent and Dlp in endosomes. Boxes indicate regions enlarged in lower panels. Scale bars represent 50 µm in normal images and 20 µm in enlarged regions. Figure 3—figure supplement 1.
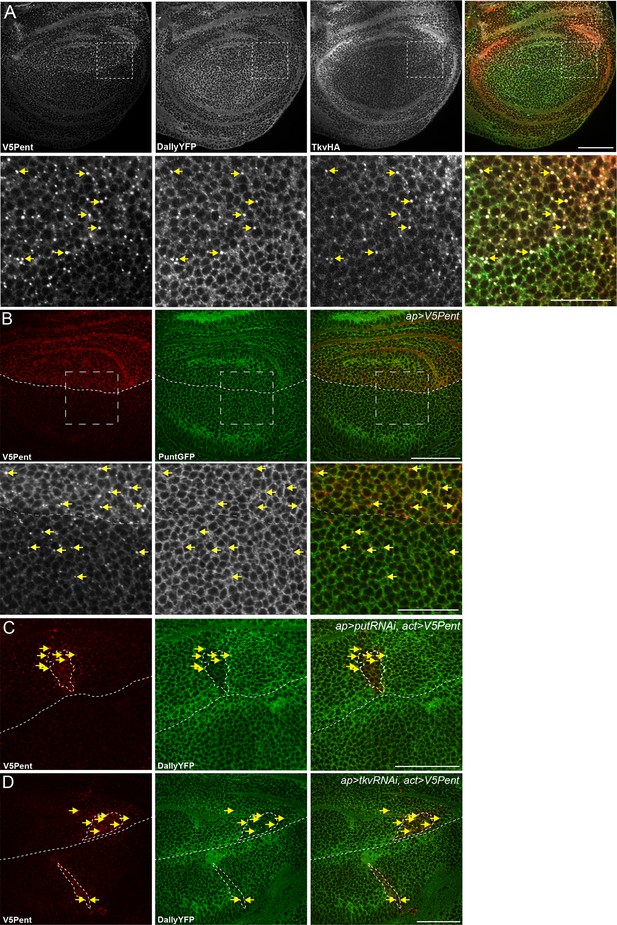
Internalisation of Dally by Pent does not require the receptor.
(A) Wing disc expressing UASV5Pent with apGal4, in a background of TkvHA and DallyYFP. Lower panels are enlargements of boxed regions. Arrows indicate Dally, Tkv and Pent co-localisation. (B) Wing disc expressing V5Pent with apGal4, in a PuntGFP background. Yellow arrows indicate dots of V5Pent that do not co-localise with PuntGFP. (C,D) Wing disc expressing Punt (C) or Tkv RNAi (D) under control of apGal4, and LexOV5Pent in clones. DallyYFP and V5Pent are still internalised when either receptor protein is knocked down and Dpp signalling is blocked. Arrows indicate co-localisation of V5Pent with DallyYFP. Scale bars are 50 µm in upper images and 20 µm in enlarged regions.
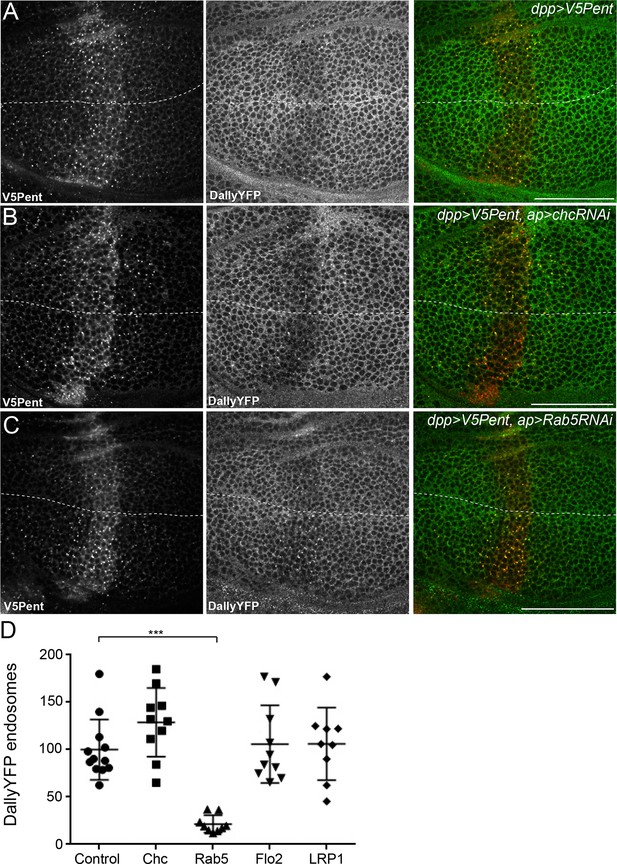
The endocytic route of V5Pent and DallyYFP.
(A) Control third instar wing disc expressing LexOV5Pent with DppLHV1 LexA driver. Reduction of DallyYFP is seen in both dorsal (above dashed line) and ventral (below dashed line) regions of the V5Pent expressing cells. V5Pent-DallyYFP endosomes are visible in both compartments. (B, C) Wing discs expressing RNAi against Chc (B) or Rab5 (C) with apGal4, restricted to 24 hr with Gal80ts. Internalisation and co-localisation of DallyYFP with V5Pent is reduced with Rab5 but not Chc RNAi. (D) Quantification of DallyYFP endosomes in RNAi experiments. Data is endosomes in dorsal, RNAi expressing compartment divided by control, ventral compartment normalised to control conditions. This shows a clear reduction when Rab5RNAi is expressed, but not with any other RNAi line. (n=9 or greater for each condition, ***represents p>0.0001, Mann-Whitney U Test). Scale bars are 50 µm in all images. See also Figure 4—figure supplement 1.
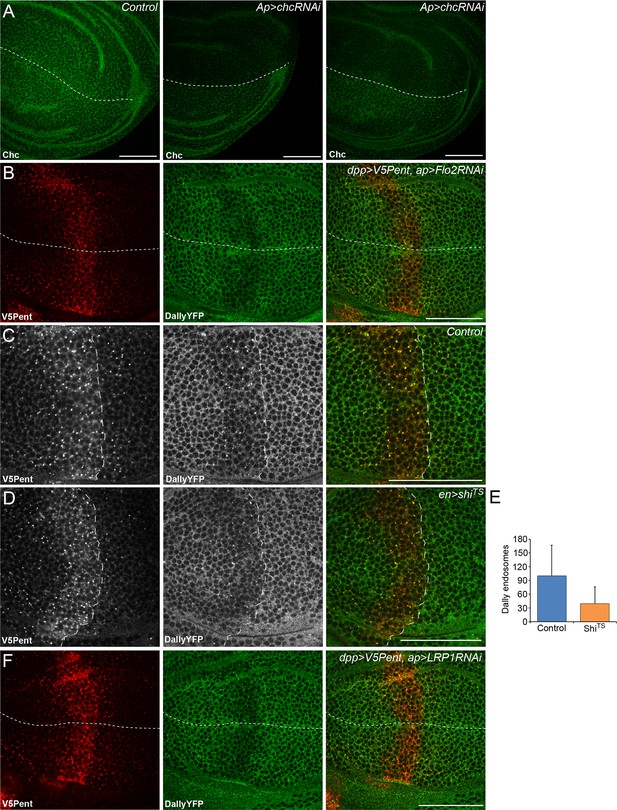
Endocytosis of Pent and Dally is clathrin and LRP1 independent.
(A) Two examples of wing discs expressing UASchcRNAi in the dorsal compartment at the Gal80ts restrictive temperature of 30°C for 24 hr. Substantial reduction in fluorescence is seen when immuno-stained with an anti-Chc antibody, compared to the control image. Dashed lines show DV boundary. (B) Wing disc expressing Flotillin 2 RNAi in the dorsal compartment and LexOV5Pent in the Dpp stripe. Knockdown of Flotillin 2 has no effect on internalisation of Pent and Dally. (C–E) Dynamin inhibits internalisation of Pent and DallyYFP. In the control disc (C) V5Pent is expressed from DppLHV1 LexA driver. V5Pent is internalised with DallyYFP in both the posterior (right of the dashed line) and anterior (left) compartments. Reduced internalisation of DallyYFP and V5Pent was seen in discs expressing ShiTS in the posterior compartment (D). Data is quantified in (E), which is the frequency of endosomes in the posterior compartment in control or when ShiTS is expressed at 30°C (n=10). (F) Wing discs expressing LRP1 RNAi in the dorsal compartment and LexOV5Pent in the Dpp stripe. Knockdown of LRP1 has no effect on internalisation of Pent and Dally. Scale bars are 50 µm.
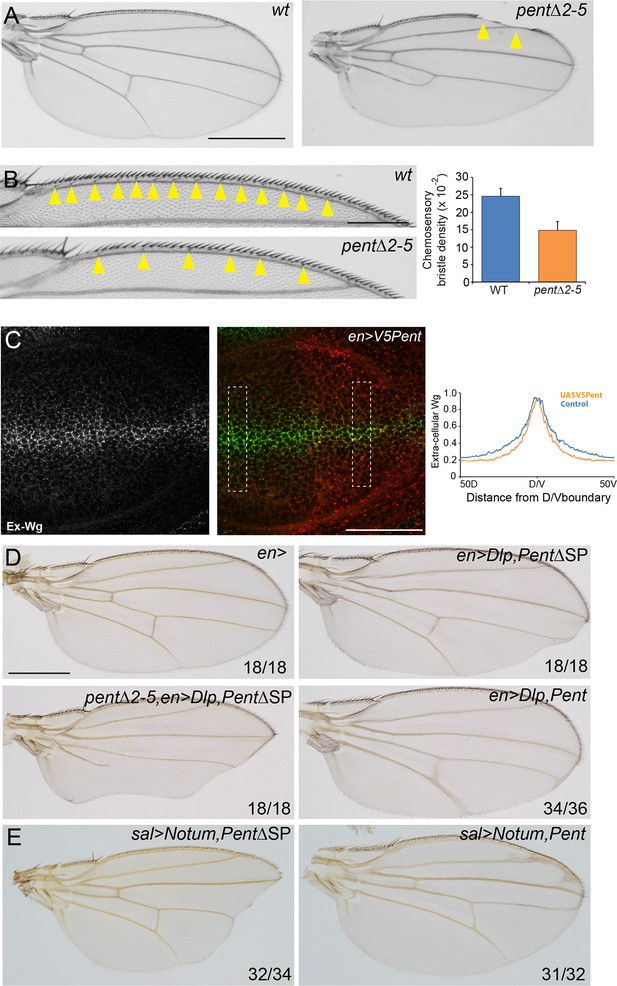
Pent modifies Wg signalling.
(A) Adult wings of WT (left) and pent△2–5 (right). 9% of pent△2–5 wings have defects in the wing margin (n=35). Scale bar is 500 µm. (B) Adult wings of wild type (upper image) and pent△2–5 (lower image) flies. Chemosensory bristles are indicated with arrows. The graph shows the density of chemosensory bristles compared to thick, outer mechanosensory bristles in WT and pent mutant (n=20). Error bars show standard deviation. (C) Third instar wing disc immuno-stained for extracellular Wg and V5Pent. Reduced staining intensity was seen in the posterior compartment which expressed V5Pent. Graph shows quantification of Pent expressing and control areas, as indicated by boxed lines in the merge panel. n=20, scale bar is 50 µm. (D) Expression of UASDlp-GFP in the posterior compartment caused scalloping of the wing which was more severe in the absence of Pent and suppressed by co-expression of Pent. (E) Expression of Notum with spaltGal4 causes severe wing defects, which are completely suppressed by co-expression of V5Pent. Numbers in (D, E) represent penetrance of displayed phenotypes. See also Figure 5—figure supplement 1.
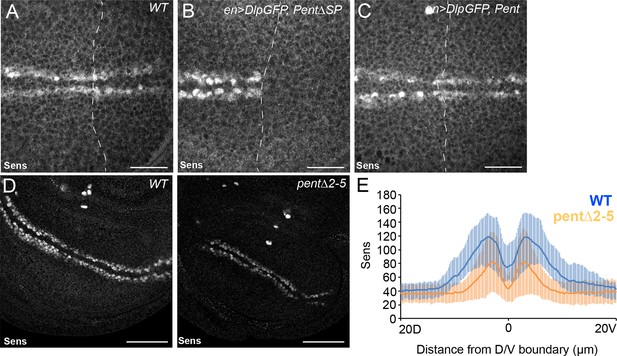
Pent modifies expression of the Wg target gene Sens.
(A–C) Control disc (A), or discs expressing DlpGFP with either V5Pent (C) or △SPV5Pent (B), which is non-functional and included to ensure equal number of UAS constructs. Expression of Pent suppresses the reduction in Sens protein normally seen when UASDlp is expressed in the posterior compartment. (D, E) WT (left) and pent△2–5 (right) discs immunostained for Sens. The level of Sens is reduced in pent△2–5, suggesting defective Wg signalling. Data are quantified in (E), where the blue line is WT and orange line is pent△2–5. Error bars show standard deviation (n=10). Scale bars are 20 µm (A–C) or 50 µm (D).
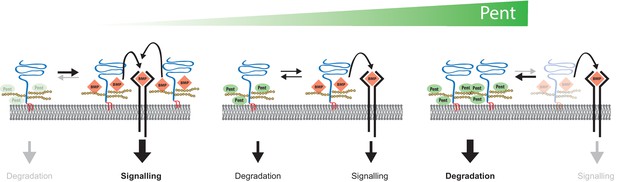
Model for Pent´s function in BMP signalling.
Pent binds to glypicans and induces their signal-independent internalisation and lysosomal degradation. In the absence of Pent (left), glypican levels on cell surfaces increase. Elevated co-receptor levels enhance local signalling but, at the same time, 'over-trap' Dpp and reduce the pool of ligand that would be available for movement and long-range dispersion. In contrast, excessive levels of Pent (as in our over-expression studies) cause a drastic depletion of surface exposed glypicans (right). As a consequence, and similar to glypican loss-of function conditions, cells fail to bind Dpp and signalling is reduced. Between these two extremes (middle), an optimal concentration of Pent ensures for glypican levels that are high enough to promote signalling but not too high to cause local trapping and 'over-consumption' of Dpp. Thus, in the context of long-range gradient formation, we suggest that adjustable levels of Pent titrate glypicans to ensure for the optimal trade-off between ligands channelled into signalling and ligands available for movement along the morphogen field (see text for details).