Single-Molecule Biophysics: The light side of the force
Single-molecule techniques are widely used to investigate how the structure of a molecule changes over time. Such measurements have advantages over standard biochemical experiments, which can only record average values measured over a large number of molecules, and traditional structural biology techniques that cannot gather dynamic information. However, a drawback of single-molecule techniques is that they typically involve measuring how just a few structural parameters – often only one, such as the end-to-end length of the molecule – vary over time. Thus, a growing trend in the field is to use hybrid instruments that combine different single-molecule techniques, and so allow more than one parameter to be observed simultaneously (Ha, 2014).
Riboswitches are regions in the non-coding regions of messenger RNA (mRNA) molecules that control the expression of the mRNA by sensing and binding to specific small 'ligand' molecules. Riboswitches comprise an aptamer region that undergoes structural transitions when a ligand binds to it, and an expression platform that regulates the expression of the mRNA in response to these shape changes. Now, in eLife, Steven Block and co-workers from Stanford University – Van Duesterberg, Irena Fischer-Hwang, Christian Perez and Daniel Hogan – combine two single-molecule techniques to investigate the structural changes that occur in the thiamine pyrophosphate (TPP) riboswitch as it unfolds and binds to TPP and other ligands (Duesterberg et al., 2015).
Most riboswitches are present only in bacteria, but the TPP riboswitch is present in all kingdoms of life. When bound to a molecule of TPP, the structure of this riboswitch resembles an inverted ‘h’ (Figure 1), with TPP docked in the region between the two 'sensor arms' of the aptamer.
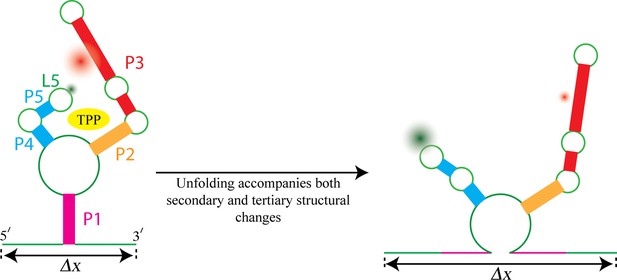
Watching the TPP riboswitch unfold.
The ligand TPP (yellow) binds to the riboswitch in the region between the two sensor arms of its aptamer, and also makes contact with each arm. There is also a long-range tertiary interaction between the loop L5 at the tip of one arm and the helix P3 in the other arm. Duesterberg, Fischer-Hwang, Perez et al. used optical trapping to apply a pulling force to the ends of the molecule and to measure the overall end-to-end extension (Δx). Simultaneously, they used smFRET to measure the separation between fluorophores (green and red fuzzy circles) placed on L5 and P3. Mechanical unfolding and the state of ligand binding affect both secondary structures and tertiary interactions. Secondary structural changes, such as unzipping one or more helices (helix P1 in this example), cause a corresponding change in Δx. Changes in long-range tertiary structure, such as the two sensor arms docking with each other, does not affect Δx, but cause a corresponding change in FRET efficiency.
Force-extension curves can be obtained by stretching individual riboswitches and measuring the end-to-end length of the molecule (Greenleaf et al., 2008). This technique has been used to monitor the mechanical unfolding of secondary structures in the TPP riboswitch, such as the various helixes that make up the aptamer (Anthony et al., 2012). Depending on how the TPP molecule is bound to the riboswitch (it can be bound strongly, weakly or not at all), these secondary structures unfold at different values of pulling force, thus producing distinct force-extension curves. However, these curves are typically insensitive to long-range tertiary interactions, such as the interactions that occur between the L5 structural loop at the tip of one sensor arm and the P3 helix at the end of the other arm (Figure 1).
Additionally, the pattern of secondary structure unfolding of the weakly bound form of the riboswitch does not change when TPP is replaced with thiamine monophosphate (TMP, which contains one phosphate group, rather than the two found in TPP) or thiamine (which contains no phosphate groups). This leaves open the possibility that the phosphates alter tertiary interactions, as these changes are undetectable in force-extension curve measurements.
A technique called smFRET has been used extensively to investigate how tertiary structures change in large molecular complexes and, more recently, in riboswitches (Lemay et al., 2006). Now, Duesterberg, Fischer-Hwang, Perez et al. have used a combined force-smFRET approach (Hohng et al., 2007; Tarsa et al., 2007) to stretch the TPP riboswitch complex while simultaneously observing both the end-to-end extension and changes in long-range tertiary interactions. smFRET measures the latter by monitoring the energy transfer between two fluorophores placed on the P3 helix and the L5 loop; the smFRET signal depends on the distance between these structural elements.
Duesterberg et al. tested TPP riboswitches that were weakly bound to one of TPP, TMP or thiamine. All three ligands showed the same pattern of force-induced unfolding of secondary structures. However, the smFRET results indicated that P3 and L5 were further apart when the riboswitch was bound to TMP or thiamine. Thus, each additional phosphate group seems to draw the sensor arms of the riboswitch closer together during ligand binding.
Further, although the two arms are more separated in the presence of bound TMP or thiamine, in all cases there was some variability in the separation distance between the arms. However, this variability is greater in the TPP-bound case. Duesterberg et al. hypothesize that the flexibility of the riboswitch’s structure when weakly bound to TPP might be essential for proper docking of the helix arms and the subsequent transition of the aptamer to the strongly bound configuration.
Both smFRET and force-extension curves have widely been used to study the folding of large biological molecules. However, this work by Duesterberg et al. represents the first example of combining the two in order to simultaneously report on secondary and tertiary structural changes during folding and ligand binding. Large-scale tertiary structural transitions are ubiquitous in biology. Applying similar approaches to the wide body of proteins and RNAs whose folding characteristics have been studied by force-extension curves alone will greatly enhance our understanding of the overall structural transitions essential for their biological functions.
The relative ease of implementation of smFRET detection has made it a technique of choice for combining with other single-molecule techniques. For instance, FRET has recently been combined with patch clamping in order to correlate structural transitions in ion channels with the flow of ionic current (Sasmal and Lu, 2014). Combining traditional single-molecule workhorses, as well as pairing single-molecule techniques with other recent advances in coarse-grained modeling and genomic techniques, will likely serve the critical need to increase the number of observable parameters available when studying molecules one at a time.
References
-
Folding energy landscape of the thiamine pyrophosphate riboswitch aptamerProceedings of the National Academy of Sciences USA 109:1485–1489.https://doi.org/10.1073/pnas.1115045109
-
Folding of the adenine riboswitchChemistry & Biology 13:857–868.https://doi.org/10.1016/j.chembiol.2006.06.010
-
Detecting force-induced molecular transitions with fluorescence resonant energy transferAngewandte Chemie International Edition 46:1999–2001.https://doi.org/10.1002/anie.200604546
Article and author information
Author details
Publication history
- Version of Record published: February 23, 2016 (version 1)
Copyright
© 2016, Basu et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,144
- views
-
- 232
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Structural Biology and Molecular Biophysics
Bacteria utilize various strategies to prevent internal dehydration during hypertonic stress. A common approach to countering the effects of the stress is to import compatible solutes such as glycine betaine, leading to simultaneous passive water fluxes following the osmotic gradient. OpuA from Lactococcus lactis is a type I ABC-importer that uses two substrate-binding domains (SBDs) to capture extracellular glycine betaine and deliver the substrate to the transmembrane domains for subsequent transport. OpuA senses osmotic stress via changes in the internal ionic strength and is furthermore regulated by the 2nd messenger cyclic-di-AMP. We now show, by means of solution-based single-molecule FRET and analysis with multi-parameter photon-by-photon hidden Markov modeling, that the SBDs transiently interact in an ionic strength-dependent manner. The smFRET data are in accordance with the apparent cooperativity in transport and supported by new cryo-EM data of OpuA. We propose that the physical interactions between SBDs and cooperativity in substrate delivery are part of the transport mechanism.
-
- Structural Biology and Molecular Biophysics
Regulated hydrolysis of the phosphoinositide phosphatidylinositol(4,5)-bis-phosphate to diacylglycerol and inositol-1,4,5-P3 defines a major eukaryotic pathway for translation of extracellular cues to intracellular signaling circuits. Members of the lipid-activated protein kinase C isoenzyme family (PKCs) play central roles in this signaling circuit. One of the regulatory mechanisms employed to downregulate stimulated PKC activity is via a proteasome-dependent degradation pathway that is potentiated by peptidyl-prolyl isomerase Pin1. Here, we show that contrary to prevailing models, Pin1 does not regulate conventional PKC isoforms α and βII via a canonical cis-trans isomerization of the peptidyl-prolyl bond. Rather, Pin1 acts as a PKC binding partner that controls PKC activity via sequestration of the C-terminal tail of the kinase. The high-resolution structure of full-length Pin1 complexed to the C-terminal tail of PKCβII reveals that a novel bivalent interaction mode underlies the non-catalytic mode of Pin1 action. Specifically, Pin1 adopts a conformation in which it uses the WW and PPIase domains to engage two conserved phosphorylated PKC motifs, the turn motif and hydrophobic motif, respectively. Hydrophobic motif is a non-canonical Pin1-interacting element. The structural information combined with the results of extensive binding studies and experiments in cultured cells suggest that non-catalytic mechanisms represent unappreciated modes of Pin1-mediated regulation of AGC kinases and other key enzymes/substrates.





