Quorum sensing control of Type VI secretion factors restricts the proliferation of quorum-sensing mutants
Abstract
Burkholderia thailandensis uses acyl-homoserine lactone-mediated quorum sensing systems to regulate hundreds of genes. Here we show that cell-cell contact-dependent type VI secretion (T6S) toxin-immunity systems are among those activated by quorum sensing in B. thailandensis. We also demonstrate that T6S is required to constrain proliferation of quorum sensing mutants in colony cocultures of a BtaR1 quorum-sensing signal receptor mutant and its parent. However, the BtaR1 mutant is not constrained by and outcompetes its parent in broth coculture, presumably because no cell contact occurs and there is a metabolic cost associated with quorum sensing gene activation. The increased fitness of the wild type over the BtaR1 mutant during agar surface growth is dependent on an intact T6SS-1 apparatus. Thus, quorum sensing activates B. thailandensis T6SS-1 growth inhibition and this control serves to police and constrain quorum-sensing mutants. This work defines a novel role for T6SSs in intraspecies mutant control.
https://doi.org/10.7554/eLife.14712.001eLife digest
Bacterial cells communicate with each other by using chemical signals. The signals allow cells living in a group to coordinate their behaviors. This cooperation can help all the cells in the group, yet scientists are puzzled about how it could evolve and persist in a population. This is because individual bacteria that essentially cheat and benefit from the cooperation of the rest of the group without contributing their fair share would have a fitness advantage.
Now, Majerczyk et al. show that a bacterium called Burkholderia thailandensis, which is commonly found in soil, poisons neighboring freeloaders to prevent such cheating. In the experiments, bacteria with mutations that allow them to ignore the chemical signals that trigger cooperation have an advantage over cooperative bacteria when the two types of bacteria are grown together in a liquid. However, the uncooperative mutants lose their advantage when they are grown on a surface where the cooperative bacteria touch them.
To understand why, Majerczyk et al. examined which genes were active in both types of cells in these situations. These experiments showed that chemical signals released by the cooperative bacteria cause them to produce both toxins and proteins that protect against these toxins. This allows the cooperative cells to poison cheaters that they come in contact with, while suffering no ill effects themselves. This allows the cooperative bacteria, via the signaling chemicals, to police cheats and reward only other cooperative bacteria. The next step will be to determine if other types of bacteria also use this policing strategy and to identify more pairs of genes that contribute to protecting the benefits of cooperation.
https://doi.org/10.7554/eLife.14712.002Introduction
Bacterial quorum sensing (QS) often controls factors that benefit cells in groups; it controls cooperative activities. A well-studied type of QS common to a number of Proteobacteria species is mediated by acyl-homoserine lactone (AHL) signals. AHLs are made by members of the LuxI family of signal synthases. The AHLs can diffuse in and out of cells. At sufficient cell densities, the AHLs accumulate to concentrations needed for expression of specific genes by binding signal receptors of the LuxR family of transcriptional regulators (for reviews of QS see (Fuqua et al., 1996)). In some bacterial species, QS controls just a few genes and in others it controls hundreds of genes. Genes for production of secreted or excreted products are overrepresented in AHL quorum sensing regulons. These extracellular products have at least in some cases been shown to serve as public goods. Any member of a group can benefit from the public good regardless of whether that individual has contributed its share of the good (West et al., 2006). There is a metabolic cost associated with QS and QS-activation of gene expression. QS control of public goods has been studied in some detail in Pseudomonas aeruginosa. Because QS activates production of extracellular proteases, QS signal receptor mutants have a fitness advantage over the wild type when growth is on milk protein as the sole carbon and energy source (Diggle et al., 2007; Sandoz et al., 2007). The receptor mutant fitness advantage presents a conundrum; how is QS-mediated cooperation preserved?
QS itself can be considered a cooperative trait, the QS signal is a public good, and QS is susceptible to exploitation by QS mutant social cheats. In P. aeruginosa, three mechanisms of QS cheater restraint have been demonstrated. The first is a direct benefit-based strategy called metabolic constraint (Schuster et al., 2013; Dandekar et al., 2012); Quorum sensing activates genes coding for public goods (such as extracellular proteases), as well as a limited set of privately utilized goods (such as a hydrolase that allows producing cells to utilize adenosine). When cells are supplied with both casein and adenosine, QS signal receptor (LasR) mutant cheats are penalized and constrained (Dandekar et al., 2012). A second mechanism has been called metabolic prudence (Xavier et al., 2011) where the QS-controlled public good is only made when it’s production is not costly. Metabolic prudence minimizes the cost of cooperation. Recently, a mechanism whereby P. aeruginosa cooperators punish or police cheats has been described. QS competent cooperators produce cyanide and are more resistant to cyanide than QS mutant cheats, and thus the cooperators can constrain cheats by slowing their growth with cyanide (Wang et al., 2015). The P. aeruginosa policing is a rare example of this sort of activity in bacteria, and because cyanide production and resistance has some semblance to a toxin-immunity-like mechanism we hypothesis that QS activation of toxin-immunity systems might have some generality as a policing mechanism where QS bacteria can control QS mutants.
We recently used RNAseq to identify genes in the Burkholderia thailandensis QS regulon (Majerczyk et al., 2014). B. thailandensis has three AHL QS systems, BtaR1-I1, BtaR2-I2 and BtaR3-I3 (Ulrich et al., 2004; Chandler et al., 2009; Duerkop et al., 2009). Together, the QS systems control hundreds of genes. The BtaR1-I1 regulon appears to be the largest as BtaR1 regulates >150 genes (Majerczyk et al., 2014). We queried the transcriptomics data and discovered that expression of several genes shown to encode Type VI secretion system (T6SS) substrates, including characterized toxins and their cognate immunity or antitoxin genes, are activated by QS. Expression of a toxin and its antitoxin results in a toxin-resistant producer cell, which can deliver toxins via the secretion apparatus via cell-cell contact to a sensitive cell thereby inhibiting growth of the sensitive cell (Russell et al., 2014). We show that T6S provides wild-type B. thailandensis with an ability to constrain BtaR1 mutants in a contact-dependent manner. This supports the hypothesis that QS activation of toxin-immunity systems can serve as a policing mechanism to control QS mutants growing among QS competent wild-type cells. Our findings also reveal yet another role for T6S in bacteria. T6S has been shown to provide a competitive interspecies or interstrain advantage, and some T6Ss have been shown to play a role in bacterial virulence (Schwarz et al., 2010; 2014). Here we describe intraspecific control of QS mutants. This provides a rationale for why T6S toxin and immunity genes might be under QS control.
Results
The BtaR1 mutant has a growth advantage over the wild type in LB broth cocultures
To assess the fitness of the B. thailandensis QS mutants in competition with their parent, we cocultured the wild type with signal blind QS mutants. We used signal-blind mutants BtaR1-, BtaR2- or BtaR3- (which each have mutations in the LuxR homolog genes btaR1, btaR2, or btaR3) because they cannot respond to signals made by QS-proficient bacteria in coculture. The starting inoculum was 99% wild type and 1% signal-blind mutant. Cocultures were transferred to fresh medium at 24 hr intervals as described in the Materials and methods (there were about 12 doublings per day), and at each interval the relative abundance of parent and mutant was determined. After 3 days in broth, the BtaR1- mutant showed an increase from 1% of the total population to 50% (Figure 1A). The BtaR2- and BtaR3- mutants did not have a growth advantage over the parent and remained at about 1% of the population throughout the experiment (Figure 1A).
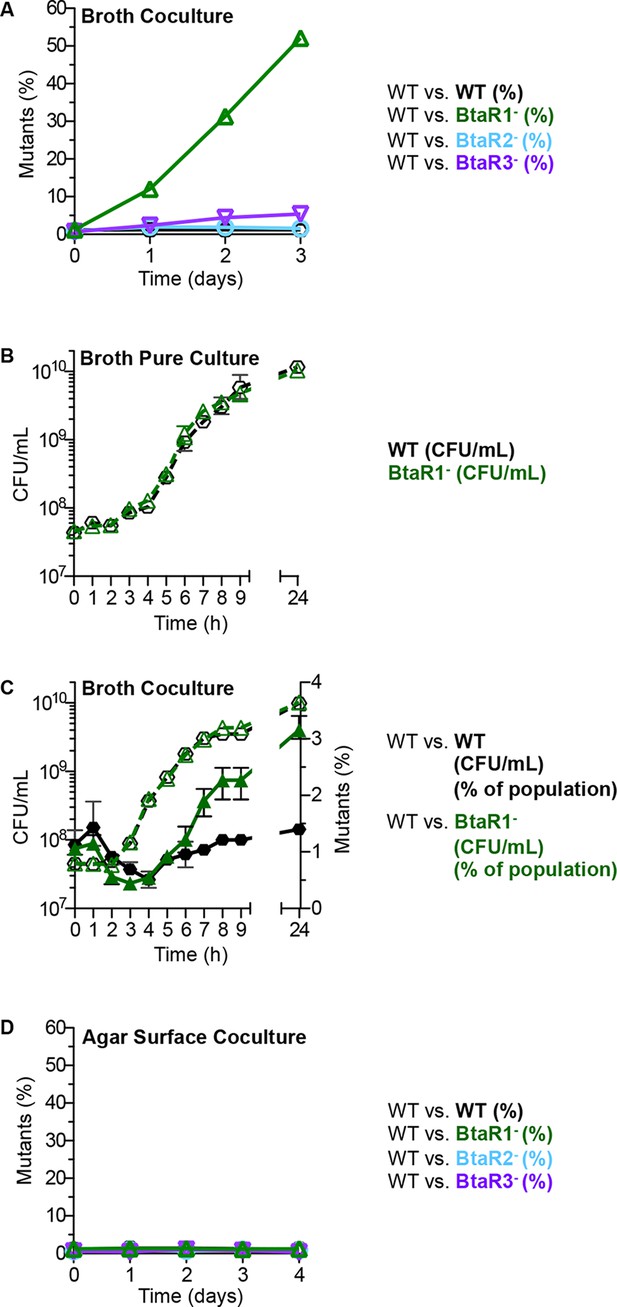
Growth of the wild type and QS mutants in LB broth and on an agar surface.
(A) In LB broth, the wild type (strain E264) was mixed with the BtaR1- mutant CM157, the BtaR2- mutant CM159 or the BtaR3- mutant CM161 at a starting mutant relative abundance of 1%. All mutants were KmR. As a control we mixed the wild type with CM218, a KmR strain derived from the wild type. Results are percent of total colonies that are KmR (% mutants). The control shows there is little cost associated with the KmR marker. (B) LB broth pure culture growth curves of CM224, a TpR wild-type strain and the KmR BtaR1- mutant. Data for Figure 1B for the statistical analysis described in the text are in the Figure 1—source data 1). (C) 24-hour LB broth coculture. The TpR marked wild type) mixed with the KmR marked wild type), or the BtaR1 mutant. KmR bacteria were started at 1% of the population and each coculture was grown in a flask for 24 hr. Bacteria were enumerated by colony counts for total coculture yield (CFU/mL) and percent KmR mutants in the population (open symbols are CFU/mL, closed symbols are percent of mutants). Bars show the mean and range of at least three biological replicates. (D) Agar surface competitions. Wild type with the BtaR1- mutant, the BtaR2- mutant or the BtaR3- mutant at a starting mutant abundance of 1%. As a control we mixed the wild type with the KmR strain derived from the wild type. Data are means of at least three biological replicates. Some ranges are not visible, as they were small and within the size of the symbols.
-
Figure 1—source data 1
Experimental data for the statistical analysis for Figure 1B.
- https://doi.org/10.7554/eLife.14712.004
Growth rates and yields of the BtaR1- mutant and the parent in pure culture were similar with a small but reproducible difference during late logarithmic phase where the parent showed slightly slower growth than the mutant (Figure 1B). At 7 hr post-inoculation in pure liquid culture, there was a reproducible and statistically significant increase in cell number for the BtaR1- mutant; the average cell yield of the wild-type parent was 1.8 x 109 CFU/mL and the BtaR1- mutant was 2.6 x 109 CFU/mL (p value=0.0041) (Figure 1B, data for this figure can be found in the Figure 1—source data 1). The slight difference in post-exponential growth rate coincides with the point during growth when we expect QS gene expression to be most evident, and when the BtaR1- mutant begins to take over the population (Figure 1C). We attribute the slight influence on growth of the parent to the cost of BtaR1-mediated QS and QS-dependent gene expression. Previous experiments showed that BtaR1 controls aproximately 164 genes in post-exponential phase, whereas BtaR2 and BtaR3 controlled 20 and 12, respectively (Majerczyk et al., 2014). We suspect that the larger BtaR1 regulon may impart a larger burden on growth than either the smaller BtaR2 or BtaR3 regulons.
The BtaR1 mutant does not have a growth advantage over the wild type in solid surface-grown cocultures
In contrast to broth cocultures where the BtaR1- mutant had a strong fitness advantage, the BtaR1- mutant had no advantage on agar plate grown cocultures at 30°C (Figure 1D) or 37°C (data not shown). The starting and final percent of the BtaR1- mutant after four daily plate transfers (about 36 doublings in total) was 1% of the total B. thailandensis population. The BtaR2- and BtaR3- mutants had no fitness disadvantage on agar plates, as was true for broth cocultures (Figure 1D).
QS regulation of known and predicted T6S effector and immunity genes
Among the many growth conditions that vary between liquid- and agar-grown cocultures is a difference in cell-to-cell contact. Cell contact is minimal during growth in broth but does occur when cells are grown on a solid agar surface. This led us to ask if QS controls contact-dependent growth inhibition systems. We previously reported that QS-1 activated the contact-dependent inhibition (CDI) system encoded by the cdiAIB genes (Majerczyk et al., 2014). However, CDI does not limit the outgrowth of the BtaR1- mutant during agar-grown cocultures. We next asked whether QS regulates other contact-dependent growth inhibitory factors. B. thailandensis contains five different T6SSs, at least one of which is used for interspecies competition (T6SS-1) (Schwarz et al., 2010). The T6SS-1 comprises a pathway that delivers effector proteins to neighboring cells upon direct cell contact. Some T6SS-1 effectors have been characterized and are toxic (Russell et al., 2014). B. thailandensis is immune to the toxins because it produces cognate immunity proteins (Russell et al., 2014). We asked if the T6SS-1 was under QS-control by querying B. thailandensis QS transcriptomics data (Majerczyk et al., 2014) for genes for the T6SS-1 apparatus or for previously identified substrates (Russell et al., 2012). Although genes for the T6SS-1 apparatus are not QS-controlled, we observed that QS activates nine genes for T6SS-1 substrates, as well as an additional eight that are either adjacent to a substrate gene, fall within the same gene cluster, or include a putative T6SS-1 immunity gene (Figure 2A).
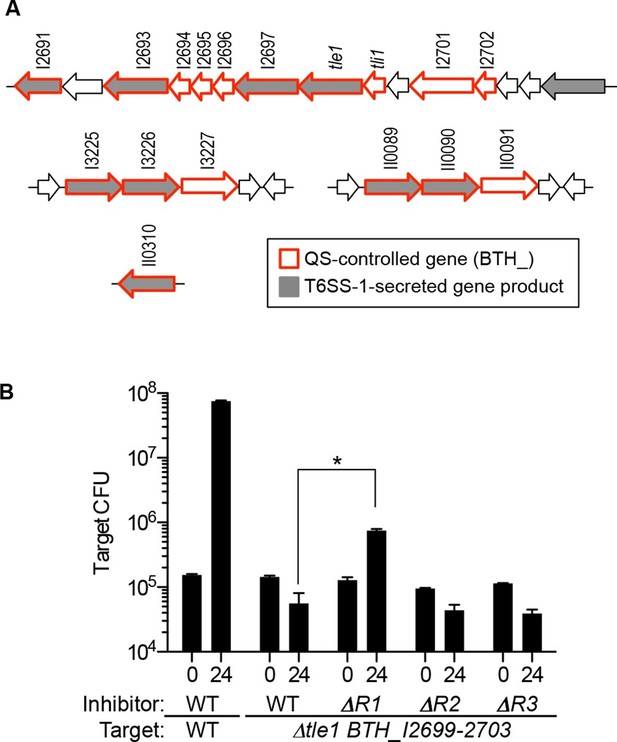
Map of QS-controlled genes for T6SS-1 substrates and solid-surface competition experiments with T6SS-1 mutants.
(A) Genes coding for known or putative T6SS-1 substrates (Russell et al., 2012) are shaded grey and genes controlled by QS (Majerczyk et al., 2014) are outlined in red. BTH_I2698 is the tle1 toxin gene and BTH_I2699 is the tli1 immunity gene (Russell et al., 2013). The neighboring genes, BTH_I2701 and BTH_I2702 are paralogs of tle1 and tli1. (B). Agar surface competition experiments. These experiments were started with a relative abundance of 10% target, CM316 TpR T6SS-1 immunity mutant (△tle1△BTH_I2699-703) or CM218 TpR wild type (WT). The inhibitor strains all carried a KmR marker; The wild type inhibitor was CM224, The BtaR1- mutant (△R1) was CM157, the BtaR2- mutant (△R2) was CM159, and the BtaR3- mutant (△R3) was CM161. Target cell abundance after one 24 hr round of colony growth was determined by plate counting. Data are from three biological replicates. The asterisk (*) indicates statistical significance between the CFU/mL measured in the WT or BtaR1 culture, as determined by an unpaired t test. Data can be found in the Figure 2—source data 1.
-
Figure 2—source data 1
Experimental data for statistical analysis of the results in Figure 2.
- https://doi.org/10.7554/eLife.14712.006
QS mutants show a T6SS-1-mediated killing defect
Ten of 15 genes in a cluster spanning BTH_I2691-BTH_I2705 were QS activated (Figure 2A). Two of these 10 QS-activated genes, BTH_I2698 (tle1) and BTH_I2699 (tli1), are a known T6S toxin and immunity pair that code for a lipase and immunity protein (Russell et al., 2013). When a B. thailandensis recipient cell does not produce the Tli1 immunity protein, Tle1 acts as a T6SS-1-delivered antibacterial factor (Russell et al., 2013). In addition to tle1 and tli1, QS activates neighboring tle1 and tli1 paralogs (BTH_I2701 and BTH_I2702) (Figure 2A). To test the hypothesis that QS activates T6SS-1-mediated killing in B. thailandensis, we competed the wild type or the signal blind QS mutants against a B. thailandensis mutant lacking tle1, tli1, and adjacent immunity paralogs (△tle1BTH_I2699-2703). Consistent with a previous publication (Russell et al., 2013), the wild type outcompeted the T6SS-1 effector and immunity mutant (Figure 2B). We also found that the BtaR1- mutant was impaired in inhibition of the effector and immunity mutant (Figure 2B). The BtaR2- and BtaR3- mutants each showed wild-type levels of inhibition (Figure 2B). We propose that the T6SS-1-mediated killing defect in the BtaR1- mutant results from its inability to fully activate expression of one or more T6S toxin genes.
The ability of wild-type B. thailandensis to compete with a BtaR1-mutant during agar growth depends on T6S
We hypothesized that the QS-activated T6SS-1 effector and immunity proteins in wild-type bacteria restrict the proliferation of BtaR1- mutants upon cell contact in cocultures. This hypothesis was tested in LB agar competition experiments with a △clpV T6SS-1 secretion apparatus mutant (T6SS-1-) and a BtaR1-T6SS-1- mutant. In this experiment, the BtaR1-T6SS-1- mutant rose in frequency from about 1% to nearly 25% of the population (Figure 3A). When the T6SS-1- mutants were complemented with clpV to restore the function of the T6SS-1 apparatus, a btaR1 mutation no longer conferred a coculture growth advantage (Figure 3A). As predicted, in broth coculture, the BtaR1- T6SS-1- mutant showed a strong fitness advantage during growth with the T6SS-1- mutant (Figure 3B). This fitness advantage was nearly identical to the fitness advantage of the BtaR1- mutant during coculture in broth with the wild-type parent (Figure 1A) and to a T6SS-1 complemented competition (Figure 3B).
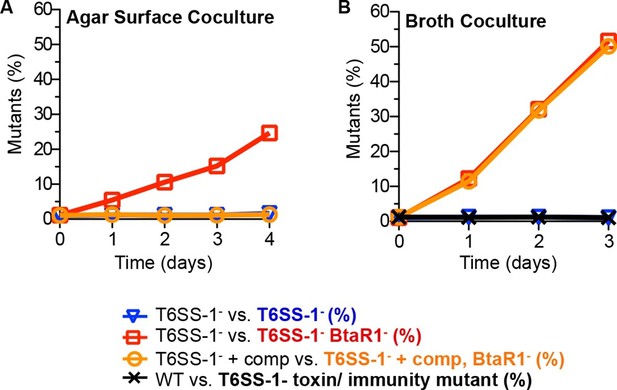
The contributions of T6SS-1 in policing BtaR1- mutants.
Competitions were on (A) LB agar plates for solid surface cocultures or in (B) LB broth for liquid cocultures. The T6SS-1-mutant (△clpV) was mixed with the TpR BtaR1- T6SS-1- mutant ES9 (open red squares) or the KmR T6SS-1- mutant ES18 (open blue downward triangle). The clpV complemented T6SS-1- mutant △clpV-comp was mixed with the clpV complemented BtaR1- T6SS-1- mutant ES16 (open orange circles). For liquid cultures, the wild type (strain E264) was mixed with CM316 Tpr T6SS-1 toxin/ immunity mutant (△tle1△BTH_I2699-703) (black crosshatch). This served as a control to demonstrate T6SS-1-mediated killing does not occur in liquid broth cocultures. Results are the mean of at least three biological replicates. Ranges were within the size of the symbols.
We reason that the parent, with an intact QS system, would express quorum-activated T6SS-1 toxin and immunity proteins, whereas the BtaR1- mutant should not fully induce its T6SS-1 effectors and immunity factors. As such, the BtaR1- mutant would be sensitive to cell contact-dependent growth inhibition or killing by the wild type. This can be considered a policing mechanism whereby QS-proficient cooperators hinder the success of individuals incapable of cooperation.
Discussion
We present evidence that QS control of T6SS-1-mediated toxicity and immunity genes allows QS competent members of B. thailandensis colonies to police against BtaR1- QS mutants. During planktonic growth in broth where T6SS-1 does not function due to limited cell contact, BtaR1- mutants have a substantial fitness advantage over wild-type cells, presumably because of the cost associated with BtaR1-dependent activities. Policing of social deviants in Myxococcus xanthus and Pseudomonas aeruginosa has been reported previously (Manhes and Velicer, 2011; Sandoz et al., 2007). Here we describe a novel mechanism, for limiting quorum sensing mutant growth in bacterial groups.
Several functions have been ascribed to T6S. B. thailandensis T6S-mediated killing is active against Proteobacteria species other than B. thailandensis and is thought to be important for interspecies competition (Schwarz et al., 2010). T6Ss occur in many species of Proteobacteria where they can provide an advantage over competing species (see Russell et al., 2014 for review). We note that in other organisms including, Pectobacterium atrosepticum (Liu et al., 2008), P. aeruginosa (Lesic et al., 2009), Aeromonas hydrophila (Khajanchi et al., 2009), Yersinia pseudotuberculosis (Zhang et al., 2011), Chromobacterium violaceum (Stauff and Bassler, 2011), Vibrio cholerae (Zheng et al., 2010), Vibrio alginolyticus, (Sheng et al., 2012), and Vibrio parahaemolyticus (Wang et al., 2013), QS regulates genes for the T6S apparatus (not the effector and immunity factors as we describe for B. thailandensis). We suspect QS regulation of the T6S apparatus might play a role in policing QS mutants during polymicrobial competitions or in other scenarios where T6S might benefit the producing cell. Furthermore, we note that other types of toxin-antitoxin systems have been reported to be under control of other non-AHL-mediated quorum sensing systems, for example the E. coli mazEF encoded toxin-antitoxin genes are controlled by a linear pentapeptide quorum sensing signal (Kolodkin-Gal et al., 2007).
Under the conditions of our experiments the BtaR1-BtaI1 QS system carries with it a metabolic cost, which is evident in broth culture competition experiments and to a lesser extent in pure broth culture grow curves. The BtaR2-BtaI2 and BtaR3-BtaI3 QS systems do not appear to carry this large cost. We describe a mechanism by which wild-type populations can mitigate the cost of BtaR1-BtaI1 gene regulation by hindering the growth of QS mutants via regulation of cell-contact-dependent toxin and immunity proteins. This is a form of microbial policing; QS-proficient bacteria are outcompeted in populations of signal blind non-cooperators in the absence of the T6S cell-contact growth inhibition system. It is interesting that BtaR1 also promotes aggregation of cells under some conditions (Chandler et al., 2009). One can imagine that this system brings cells into close proximity where they can resist infiltration by other strains and species while at the same time protect themselves against social deviant BtaR1 mutant bacteria (Figure 4).
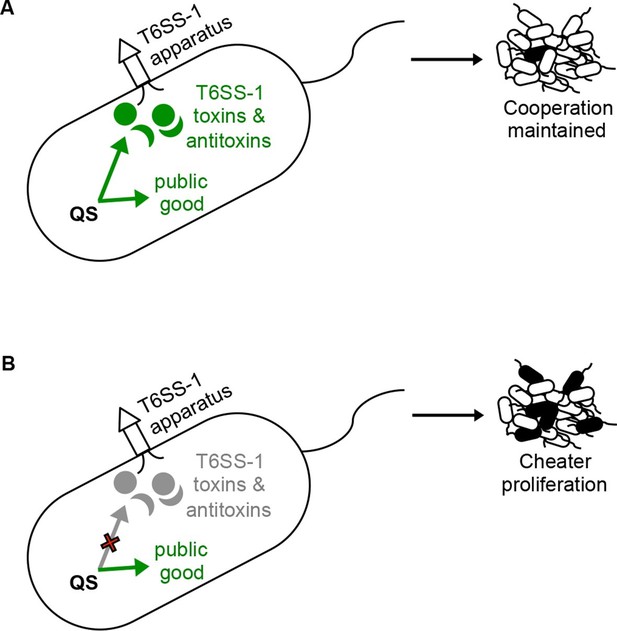
A policing model where QS control of T6S contact-dependent killing (effector and immunity proteins) enforces cooperation.
(A) The bacterium on the left depicts a situation where QS co-regulates a public good with the T6SS-1 effector and immunity factors. Under these conditions, QS-proficient bacteria (cooperators, shown as white cells) limit the proliferation of a QS mutant (potential cheater, black cell) in a population. (B) In this situation, QS no longer co-regulates public good production with the T6SS-1 killing mechanism. Without co-regulation of the killing mechanism, QS mutants (potential cheaters, black cells) outcompete the wild type cooperators (white cells).
Materials and methods
Bacterial strains, plasmids, and growth conditions
Request a detailed protocolBacterial strains and plasmids are listed in Table 1. Bacteria were grown in Luria-Bertani (LB) broth (10 g tryptone, 5 g yeast extract, and 5 g NaCl per liter) supplemented with 50 mM morpholinepropanesulfonic acid (MOPS) buffer (pH 7.0) or on LB MOPS agar plates (LB MOPS plus 2% agar). Antibiotics were added at the following concentrations as appropriate: for Escherichia coli, 100 μg/mL trimethoprim (Tp), 25 μg/mL zeocin (Zeo), and 100 μg/mL ampicillin (Ap) and for B. thailandensis, 100 μg/mL Tp, 2 mg/mL Zeo, and 1 mg/mL Km. Except where indicated, bacteria were grown in broth at 37°C with shaking or on agar plates at 30°C.
Bacterial strains and plasmids used in this study
| Bacterial strain or plasmid | Genotype or descriptiona | Source | |
|---|---|---|---|
| Bacterial strains | |||
| DH10B | E. coli cloning vehicle | Invitrogen | |
| E264 | Wild-type B. thailandensis | (Brett et al., 1998) | |
| JBT107 | E264 △btaR1 | (Chandler et al., 2009) | |
| JBT108 | E264 △btaR2 | (Chandler et al., 2009) | |
| JBT109 | E264 △btaR3 | (Chandler et al., 2009) | |
| CM157 | JBT107 glmS1 attn7::Km; KmR | This study | |
| CM159 | JBT108 glmS1 attn7::Km; KmR | This study | |
| CM161 | JBT109 glmS1 attn7::Km; KmR | This study | |
| CM218 | E264 glmS1 attn7::Km; KmR | This study | |
| CM224 | E264 glmS1 attn7::Tp; TpR | This study | |
| BT03399 | E264 btaR1139::ISlacZ/PrhaBo-Tp/FRT; TpR | (Gallagher et al., 2013) | |
| △clpV | E264 △BTH_I2958 | (Schwarz et al., 2010) | |
| △clpV-comp | △clpV att Tn7-miniTn7T-Tp-S12-BTH_I2958 | (Schwarz et al., 2010) | |
| ES9 | △clpV btaR1139::ISlacZ/PrhaBo-Tp/FRT; TpR | This study | |
| ES18 | △clpV glmS1 attn7::Tp; TpR | This study | |
| ES14 | △clpV btaR1139::ISlacZ/PrhaBo scar; TpS | This study | |
| ES15 | ES14 glmS1 attn7::PS12-clpV, Tp; TpR | This study | |
| ES16 | ES15 glmS2 attn7::Km; TpR, KmR | This Study | |
| △tle1△BTH_I2699-703 | E264 △BTH_I2698-BTH_I2703 | (Russell et al., 2013) | |
| CM316 | △tle1 △12699-2700 glmS1 attn7::Tp; TpR | This study | |
| Plasmids | |||
| pTNS2 | R6K replicon TnsABC+D vector | (Choi et al., 2005) | |
| pUC18T-mini-Tn7T-Tp | Cloning vector | (Choi and Schweizer, 2006) | |
| pUC18T-mini-Tn7T-Km-FRT | Cloning vector | (Choi et al., 2008) | |
| pFLPe2 | Flp recombinase-expressing vector; ZeoR | (Choi et al., 2008) | |
| pUC18T-miniTn7T-Tp-S12-BTH_I2958 | clpV complementation contruct (PS12-clpV) | (Schwarz et al., 2010) | |
Mutant construction
Request a detailed protocolTo generate Tp-resistant mutants we transformed strains E264, △clpV, and △tleBT △12699–2700, with pTNS2 and pUC18T-mini-Tn7T-Tp as described elsewhere (Majerczyk et al., 2014) to make CM224 (E264 glmS1 attn7::Tp), ES18 (△clpV glmS1 attn7::Km), and CM316 (△tleBT△12699-2700glmS1 attn7::Tp), respectively. To generate Km-resistant mutants we transformed B. thailandensis strains JBT107, JBT109, JBT110, E264, and CM183 with pTNS2 and pUC18T-mini-Tn7T-km to generate CM157 (△btaR1 glmS1 attn7::Km), CM159 (△btaR2 glmS1 attn7::Km), and CM161 (△btaR3 glmS1 attn7::Km). Genomic DNA (gDNA) from strain BT03399 was used to introduce a btaR1 mutation into △clpV to make ES9 (△clpV btaR1139::ISlacZ/PrhaBo-Tp/FRT; TpR) by natural transformation as previously described (Thongdee et al., 2008). The trimethoprim resistance cassette was removed from strain ES9 to make strain ES14 (△clpV btaR1139::ISlacZ/PrhaBo scar) by using pFLPe2 as previously described (Choi et al., 2008). The clpV complementation construct pUC18T-miniTn7T-Tp-S12-BTH_I2958 (Schwarz et al., 2010) was introduced into ES14 by electrotransformation with pTNS2 as described elsewhere (8) to generate ES15 (ES14 glmS1 attn7::PS12-clpV, Tp). ES16 (ES15 glmS2 attn7::Km; TpR, KmR) was then made by transforming ES15 with pTNS2 and pUC18T-mini-Tn7T-km. We used PCR to confirm that the antibiotic resistance markers and complementation construct were inserted at the attn7 site near glmS1 or glmS2 in all of the constructs..
LB broth growth experiments
Request a detailed protocolFor single strain growth curves bacteria were grown in 15 mL of broth in 125-mL flasks with an overnight culture as inoculum to a starting optical density at 600 nm of 0.05. We sampled cultures every hour and determined cell numbers by plate counting. For competition experiments we used inocula from overnight cultures at a ratio of 99:1 as indicated. As above, the starting optical densities were 0.05 in a volume of 15 mL in a 125-mL flask. Abundances of each strain were determined by plate counting on selective media.
Long-term B. thailandensis competition experiments
Request a detailed protocolLogarithmic phase cultures of each competing strain were diluted in fresh LB medium to an optical density of 0.2. The diluted cultures were then mixed at a 99:1 or 9:1 ratio where indicated and 20 μl of the mixture was spotted on an agar plate or inoculated into 4 mL of broth in a 16 mm test tube. The solid surface-grown cocultures were incubated at 30°C and the liquid-grown cocultures were incubated with shaking at 37°C to minimize aggregation. At 24 hr intervals cells were harvested, counted and used to inoculate a new broth or plate. Colonies on plates were scraped from the agar surface, suspended in phosphate buffer (pH 7.0), and subjected to water bath sonication for 10 min to disrupt aggregates. The cells in phosphate buffer were diluted to an optical density of 0.2. Twenty μl of the density-adjusted cell suspension was spotted onto an agar plate for another round of competition. Bacteria grown in broth were similarly diluted and 20 μL of the density adjusted cell suspension was inoculated into a test tube with 4 mL broth. At each transfer cells of each of the competing strains were enumerated by selective plate counting.
Data availability
-
Burkholderia thailandensis E264 Burkholderia thailandensis E264 Transcriptome or Gene expressionPublicly available at BioProject of NCBI (accession no: PRJNA233628).
References
-
Burkholderia thailandensis sp. nov., a Burkholderia pseudomallei-like speciesInternational Journal of Systematic Bacteriology 48:317–320.https://doi.org/10.1099/00207713-48-1-317
-
Mutational analysis of Burkholderia thailandensis quorum sensing and self-aggregationJournal of Bacteriology 191:5901–5909.https://doi.org/10.1128/JB.00591-09
-
A Tn7-based broad-range bacterial cloning and expression systemNature Methods 2:443–448.https://doi.org/10.1038/nmeth765
-
Genetic tools for select-agent-compliant manipulation of Burkholderia pseudomalleiApplied and Environmental Microbiology 74:1064–1075.https://doi.org/10.1128/AEM.02430-07
-
Quorum-sensing control of antibiotic synthesis in Burkholderia thailandensisJournal of Bacteriology 191:3909–3918.https://doi.org/10.1128/JB.00200-09
-
Census and consensus in bacterial ecosystems: the LuxR-LuxI family of quorum-sensing transcriptional regulatorsAnnual Review of Microbiology 50:727–751.https://doi.org/10.1146/annurev.micro.50.1.727
-
Global analysis of the Burkholderia thailandensis quorum sensing-controlled regulonJournal of Bacteriology 196:1412–1424.https://doi.org/10.1128/JB.01405-13
-
Experimental evolution of selfish policing in social bacteriaProceedings of the National Academy of Sciences of the United States of America 108:8357–8362.https://doi.org/10.1073/pnas.1014695108
-
Type VI secretion system effectors: Poisons with a purposeNature Reviews Microbiology 12:137–148.https://doi.org/10.1038/nrmicro3185
-
Social cheating in Pseudomonas aeruginosa quorum sensingProceedings of the National Academy of Sciences of the United States of America 104:15876–15881.https://doi.org/10.1073/pnas.0705653104
-
Acyl-homoserine lactone quorum sensing: From evolution to applicationAnnual Review of Microbiology 67:43–63.https://doi.org/10.1146/annurev-micro-092412-155635
-
Quorum sensing in Chromobacterium violaceum: DNA recognition and gene regulation by the CviR receptorJournal of Bacteriology 193:3871–3878.https://doi.org/10.1128/JB.05125-11
-
Targeted mutagenesis of Burkholderia thailandensis and Burkholderia pseudomallei through natural transformation of PCR fragmentsApplied and Environmental Microbiology 74:2985–2989.https://doi.org/10.1128/AEM.00030-08
-
Quorum sensing and policing of Pseudomonas aeruginosa social cheatersProceedings of the National Academy of Sciences of the United States of America 112:2187–2191.https://doi.org/10.1073/pnas.1500704112
-
Social evolution theory for microorganismsNature Reviews Microbiology 4:597–607.https://doi.org/10.1038/nrmicro1461
-
A molecular mechanism that stabilizes cooperative secretions in Pseudomonas aeruginosaMolecular Microbiology 79:166–179.https://doi.org/10.1111/j.1365-2958.2010.07436.x
-
Quorum sensing and a global regulator TsrA control expression of type VI secretion and virulence in Vibrio choleraeProceedings of the National Academy of Sciences of the United States of America 107:21128–21133.https://doi.org/10.1073/pnas.1014998107
Article and author information
Author details
Funding
National Institute of General Medical Sciences (GM59026)
- E Peter Greenberg
National Institute of Allergy and Infectious Diseases (AI057141)
- E Peter Greenberg
Guangdong Province Grant (2013S034)
- E Peter Greenberg
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
This research was supported by USPHS grants AI057141 and GM59026, and an Innovative Team Program of Guangdong Province grant 2013S034. We thank Alistair Russell and Joseph Mougous for helpful discussions.
Version history
- Received: January 26, 2016
- Accepted: April 8, 2016
- Version of Record published: May 16, 2016 (version 1)
Copyright
© 2016, Majerczyk et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,277
- views
-
- 547
- downloads
-
- 71
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Microbiology and Infectious Disease
- Structural Biology and Molecular Biophysics
African trypanosomes replicate within infected mammals where they are exposed to the complement system. This system centres around complement C3, which is present in a soluble form in serum but becomes covalently deposited onto the surfaces of pathogens after proteolytic cleavage to C3b. Membrane-associated C3b triggers different complement-mediated effectors which promote pathogen clearance. To counter complement-mediated clearance, African trypanosomes have a cell surface receptor, ISG65, which binds to C3b and which decreases the rate of trypanosome clearance in an infection model. However, the mechanism by which ISG65 reduces C3b function has not been determined. We reveal through cryogenic electron microscopy that ISG65 has two distinct binding sites for C3b, only one of which is available in C3 and C3d. We show that ISG65 does not block the formation of C3b or the function of the C3 convertase which catalyses the surface deposition of C3b. However, we show that ISG65 forms a specific conjugate with C3b, perhaps acting as a decoy. ISG65 also occludes the binding sites for complement receptors 2 and 3, which may disrupt recruitment of immune cells, including B cells, phagocytes, and granulocytes. This suggests that ISG65 protects trypanosomes by combining multiple approaches to dampen the complement cascade.
-
- Microbiology and Infectious Disease
In the Firmicutes phylum, GpsB is a membrane associated protein that coordinates peptidoglycan synthesis with cell growth and division. Although GpsB has been studied in several bacteria, the structure, function, and interactome of Staphylococcus aureus GpsB is largely uncharacterized. To address this knowledge gap, we solved the crystal structure of the N-terminal domain of S. aureus GpsB, which adopts an atypical, asymmetric dimer, and demonstrates major conformational flexibility that can be mapped to a hinge region formed by a three-residue insertion exclusive to Staphylococci. When this three-residue insertion is excised, its thermal stability increases, and the mutant no longer produces a previously reported lethal phenotype when overexpressed in Bacillus subtilis. In S. aureus, we show that these hinge mutants are less functional and speculate that the conformational flexibility imparted by the hinge region may serve as a dynamic switch to finetune the function of the GpsB complex and/or to promote interaction with its various partners. Furthermore, we provide the first biochemical, biophysical, and crystallographic evidence that the N-terminal domain of GpsB binds not only PBP4, but also FtsZ, through a conserved recognition motif located on their C-termini, thus coupling peptidoglycan synthesis to cell division. Taken together, the unique structure of S. aureus GpsB and its direct interaction with FtsZ/PBP4 provide deeper insight into the central role of GpsB in S. aureus cell division.






