Sam68/KHDRBS1 is critical for colon tumorigenesis by regulating genotoxic stress-induced NF-κB activation
Figures
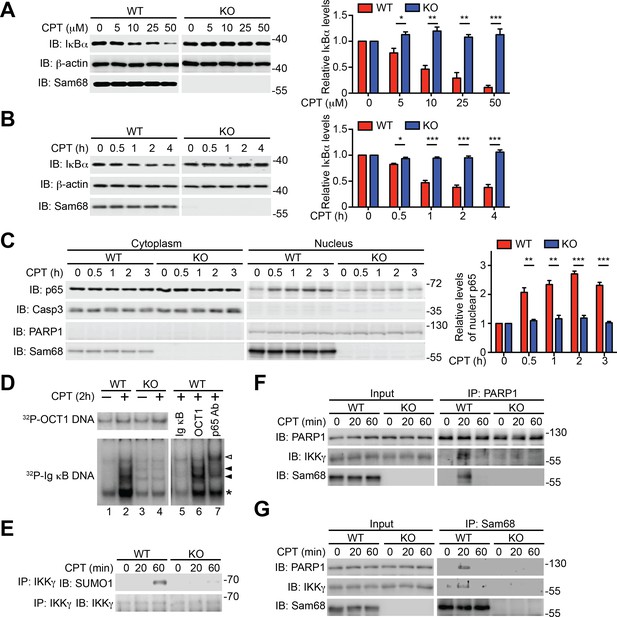
Sam68 is required for the nuclear-initiated NF-κB signaling in response to DNA damage.
(A and B) Whole cell lysates from wild-type (WT) and Sam68 knockout (KO) mouse embryonic fibroblasts (MEFs) treated with indicated concentrations of CPT for 2 hr (A) or 10 μM of CPT for indicated periods (B) were immunoblotted (IB) for IκBα and Sam68, with β-actin as a loading control. Right, the IκBα levels, normalized to β-actin and untreated controls, were quantified from three independent experiments. (C) Cytosolic and nuclear fractions derived from WT and Sam68 KO MEFs stimulated with 10 μM of CPT for indicated periods were IB for indicated proteins. Caspase-3 (Casp3) and PARP1 served as loading controls and cytosolic and nuclear markers, respectively. Right, the p65 levels in the nucleus, normalized to PARP1 and untreated controls, were quantified from three independent experiments. (D) Nuclear extracts of WT and Sam68 KO MEFs treated with (+) or without (−) CPT (10 μM, 2 hr) were analyzed by EMSA with 32P-labeled immunoglobin (Ig) κB or OCT1 oligonucleotides. In some cases, EMSA was performed in the presence of 100-fold unlabeled Ig κB or OCT1 oligonucleotide competitors (lanes 5–6) or p65 antibody (Ab) (lane 7). Ig κB DNA binding complexes are labeled with filled triangles, and the supershifted band and nonspecific band are labeled with an open triangle and an asterisk, respectively. (E) WT and Sam68 KO MEFs were stimulated with 10 μM of CPT for indicated periods, and immunoprecipitants (IP) with IKKγ antibody were immunoblotted for indicated proteins. (F and G) Whole cell lysates (Input) from WT and Sam68 KO MEFs stimulated with 10 μM of CPT for indicated periods were IB directly or after IP with PARP1 antibody (F) or Sam68 antibody (G) for indicated proteins. Data are representative of at least three independent experiments. Results in (A, B and C) are expressed as mean and s.e.m. *p<0.05, **p<0.01, ***p<0.001 by Student’s t tests.
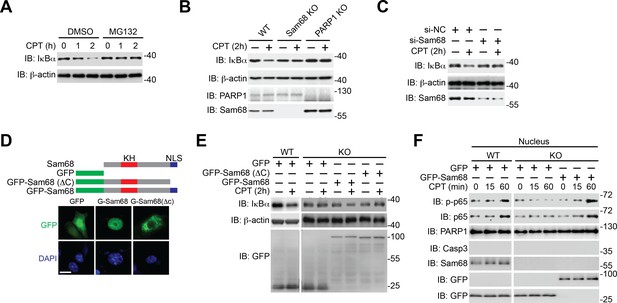
Sam68 is critical for genotoxic stress-induced IκBα degradation.
(A) Wild-type (WT) mouse embryonic fibroblasts (MEFs), pretreated with DMSO or 10 μM of MG132 for 2 hr, were stimulated with 10 μM of CPT for indicated periods. Whole cell lysates were derived and immunoblotted (IB) for IκBα, with β-actin as a loading control. (B) Whole cell lysates from WT, Sam68 knockout (Sam68 KO), and PARP1 knockout (PARP1 KO) MEFs treated with (+) or without (−) 10 μM of CPT for 2 hr were IB for IκBα, PARP1, and Sam68, with β-actin as a loading control. (C) WT MEFs transiently transfected with either non-specific control (si-NC) small interference RNA (siRNA) or siRNA targeting Sam68 (si-Sam68 Sam68) were treated with (+) or without (−) 10 μM of CPT for 2 hr, and whole cell lysates were derived and IB for IκBα and Sam68, with β-actin as a loading control. (D) Schematic diagram of the full-length (residues 1–443) and truncated mutant (ΔC lacks residues 347–443) of Sam68 fused with GFP. The hnRNP K homology (KH) domain and nuclear localization signal (NLS) are labeled in red and blue, respectively. Immunofluorescence micrographs of MEFs transiently transfected with GFP or indicated GFP fusion proteins, with nuclei counterstained by DAPI. Scale bar, 10 μm (bottom). (E) WT and Sam68 KO MEFs transiently transfected with GFP or indicated GFP fusion proteins were stimulated with (+) or without (−) CPT (10 μM, 2 hr), and whole cell lysates were derived and IB for IκBα and GFP, with β-actin as a loading control. (F) WT and Sam68 KO MEFs transiently transfected with GFP or GFP-Sam68 fusion protein were stimulated with 10 μM of CPT for indicated time periods. Nuclear fractions were derived and IB for indicated proteins. Caspase-3 (Casp3) and PARP1 served as loading controls and cytosolic and nuclear markers, respectively. p-p65, Ser536 phosphorylated p65.
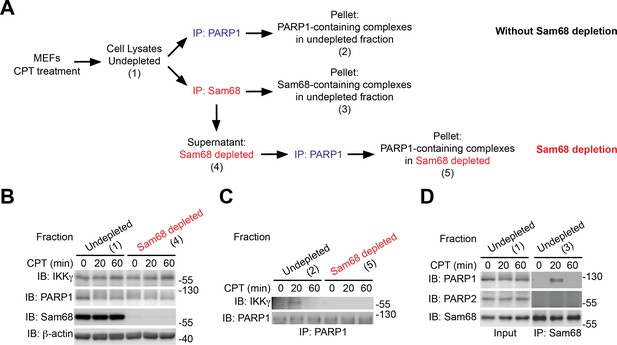
Sam68 complexes with PARP1 and IKKγ during the cellular response to genotoxic stress.
(A) Scheme of Sam68 depletion of in CPT-stimulated wild-type mouse embryonic fibroblasts (MEFs). MEFs were stimulated with 100 μM of CPT for 0, 20, and 60 min. Under the Sam68 depletion condition, cell lysates were precleared with anti-Sam68 antibody (IP: Sam68) in order to remove all the Sam68-containing complexes. The remaining lysates (Fraction Sam68 depleted) were immunoprecipitated (IP) with an anti-PARP1, and the interaction between PARP1 and IKKγ was evaluated by immunoblot (IB). Under the condition without Sam68 depletion, cell lysates were directly IP with anti-PARP1 or anti-Sam68 antibody. (B) Identical aliquots of indicated fractions were IB for indicated proteins, with β-actin as a loading control. (C) The indicated fractions from either Sam68-depleted or –undepleted condition were IP with anti-PARP1. The immunoprecipitants were separated and IB for PARP1 and IKKγ. (D) The Sam68-undepleted whole cell lysates were IB directly or after IP with anti-Sam68 antibody for the indicated proteins.
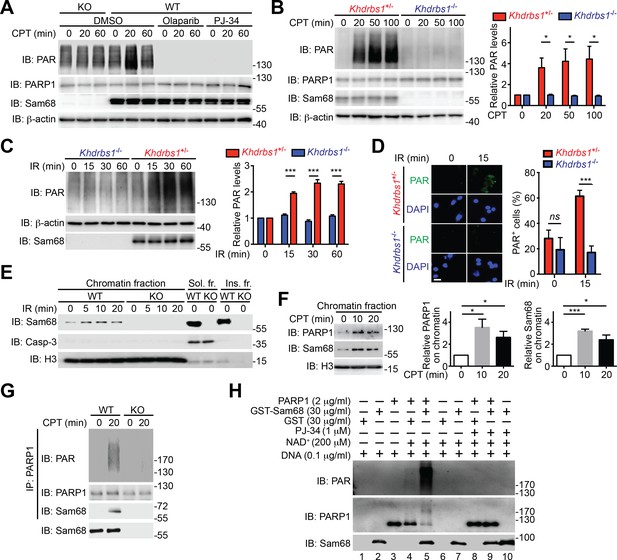
Sam68 facilitates PARP1-catalyzed PARylation in response to DNA damage.
(A) Wild-type (WT) and Sam68 knockout (KO) mouse embryonic fibroblasts (MEFs) pretreated with Olaparib (10 μM), PJ-34 (10 μM), or DMSO for 1 hr, were stimulated with 10 μM of CPT for indicated periods, and whole cell lysates were immunoblotted (IB) for indicated proteins, with β-actin as a loading control. (B and C) Primary colonic epithelial cells (CECs) isolated from Khdrbs1+/- and Khdrbs1-/- mice were stimulated with 10 μM of CPT (B) or 10 Gy of γ-irradiation (IR) (C). Whole cell lysates were derived at indicated periods post stimulation and IB as in (A). Right, the PAR levels, normalized to β-actin and untreated controls, were quantified from three independent experiments. (D) Immunofluorescence micrographs of PARylated proteins (PAR) in CECs treated as in (C), with nuclei counterstained by DAPI. Scale bar, 10 μm. Percentage of CECs (>100 cells from 5–8 random fields) with PAR staining was quantified (right). (E) WT and Sam68 KO MEFs were γ-irradiated (IR) at 10 Gy and the chromatin, soluble (Sol. fr.), and insoluble (Ins. fr.) subcellular fractions were derived at indicated time points post γ-irradiation and IB for Sam68, Caspase-3 (Casp-3), and Histone H3 (H3). (F) WT MEFs were stimulated with 10 μM of CPT for indicated periods and the chromatin fractions were derived and IB as in (E). Right, the Sam68 and PARP1 levels in the chromatin fractions, normalized to H3 and untreated controls, were quantified from three independent experiments. (G) Whole cell lysates from WT and Sam68 KO MEFs stimulated with 10 μM of CPT for 20 min, were IB directly or after immunoprecipitation (IP) with PARP1 antibody for indicated proteins. (H) Recombinant PARP1 protein was incubated in reaction buffer containing damaged DNA or with purified GST or GST-Sam68 protein in the presence or absence of NAD+ or the PARP1 inhibitor PJ-34, as indicated. The reaction mixture was separated by SDS-PAGE and subjected to IB with the PAR, PARP1, and Sam68 antibodies. Results in (B, C, D and F) are expressed as mean and s.e.m. ns, non-significant difference and , *p<0.05, ***p<0.001 by Student’s t tests. Data are representative of at least three independent experiments.
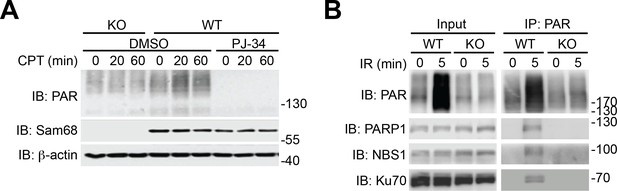
Sam68 deletion attenuates genotoxic stress-stimulated PARylation.
(A) Wild-type (WT) and Sam68 knockout (KO) mouse embryonic fibroblasts (MEFs) pretreated with PJ-34 (10 μM) or DMSO for 1 hr, were stimulated with 10 μM of CPT for indicated periods, and whole cell lysates were immunoblotted (IB) for indicated proteins, with β-actin as a loading control. (B) WT and Sam68 KO MEFs were γ-irradiated (IR) at 10 Gy. Whole cell lysates (Input) derived at 0 or 5 min post γ-irradiation were IB directly or after immunoprecipitation (IP) with PAR antibody for indicated proteins.
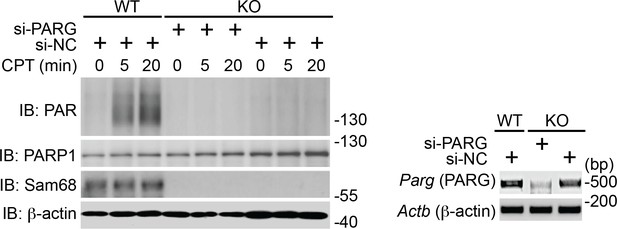
Down-regulation of PARG does not restore DNA damage-induced PAR production in Sam68 KO MEFs.
Wild-type (WT) and Sam68 knockout (Sam68 KO) mouse embryonic fibroblasts (MEFs) transfected with non-specific control (si-NC) or PARG-specific (si-PARG) small interference RNA, as indicated, were stimulated with 100 μM of CPT for 0, 5, and 20 min. Whole cell lysates were derived and immunoblotted (IB) for indicated proteins, with β-actin as a loading control. The down-regulation of PARG in indicated cells was evaluated by semi-quantitative RT-PCR (Right).
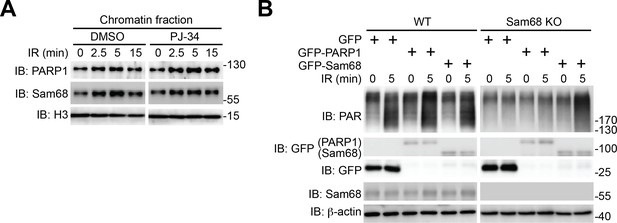
Sam68 functions upstream of PARP1 in cellular response to genotoxic stresses.
(A) Wild-type mouse embryonic fibroblasts (MEFs), pretreated with PJ-34 (10 μM) or DMSO for 1 hr, were γ-irradiated (IR) at 10 Gy. The chromatin fractions derived at indicated time points post γ-irradiation were immunoblotted (IB) for PARP1 and Sam68, with Histone H3 (H3) as a loading control. (B) Wild-type (WT) and Sam68 knockout (KO) MEFs expressing indicated GFP or GFP-fusion proteins were IR at 10 Gy. Whole cell lysates were derived at indicated time points post γ-irradiation and IB for the indicated proteins.
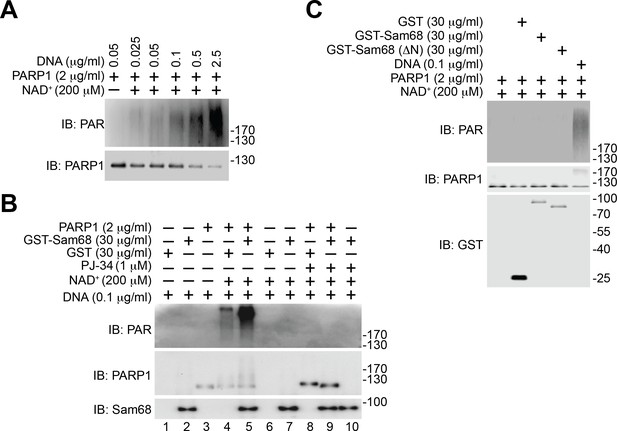
Sam68-stimulated PARP1 PARylation is DNA dependent.
(A) Recombinant PARP1 protein was incubated in reaction buffer containing NAD+ and damaged DNA as indicated. The reaction mixture was separated by SDS-PAGE and subjected to immunoblot (IB) with the PAR and PARP1. (B) Recombinant PARP1 protein was incubated in reaction buffer containing damaged DNA or with purified GST or GST-Sam68 protein in the presence or absence of NAD+ or the PARP1 inhibitor PJ-34, as indicated. The reaction mixture was separated by SDS-PAGE and subjected to IB with the PAR, PARP1, and Sam68 antibodies. (C) Recombinant proteins were incubated in reaction buffer in the presence or absence of damaged DNA and NAD+, as indicated. The reaction mixture was separated by SDS-PAGE and IB for indicated proteins.
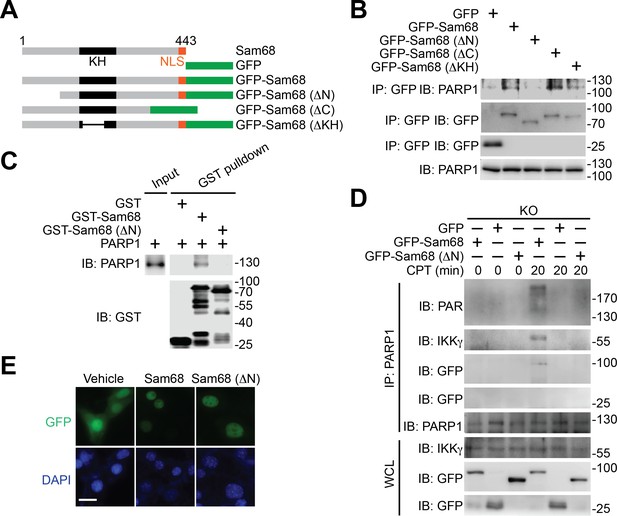
N-terminus of Sam68 is crucial for the Sam68-PARP1 interaction and genotoxic stress-induced NF-κB signalosome assembly.
(A) Schematic diagram of Sam68 protein (residues 1–443), full-length or indicated mutants (ΔN lacks residues 1–102, ΔC lacks 347–443, and ΔKH lacks 165–224) fused with GFP. KH, The hnRNP K homology (KH) domain and nuclear localization signal (NLS) are labeled in black and orange, respectively. (B) Whole cell lysates from HEK293T cells expressing indicated GFP or GFP-fusion proteins were IB directly or after IP with GFP antibody for indicated proteins. (C) Whole cell lysate (Input) containing recombinant PARP1 were IB directly or after pulldown with indicated GST or GST-fusion proteins for indicated proteins. (D) Sam68 KO MEFs expressing GFP, GFP-Sam68, or GFP-Sam68 (ΔN) proteins were stimulated with 10 μM of CPT for indicated periods, and the derived whole cell lysates (WCL) were IB directly or after IP with PARP1 antibody for indicated proteins. (E) Immunofluorescence micrographs of Sam68 KO MEFs expressing GFP (Vehicle), GFP-Sam68 (Sam68), or GFP-Sam68 (ΔN) proteins, with nuclei counterstained by DAPI. Scale bar, 10 μm. Data in (B–E) are representative of at least three independent experiments.
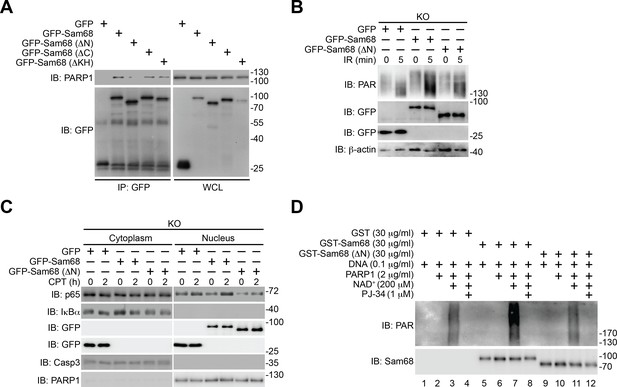
The N-terminus-mediated Sam68-PARP1 interaction is critical for DNA damage-induced PARylation and NF-κB activation.
(A) Whole cell lysates (WCL) from HEK293T cells expressing GFP or indicated GFP-fusion proteins were immunoblotted (IB) directly or after immunoprecipitation (IP) with GFP antibody for indicated proteins. (B) Sam68 knockout (KO) mouse embryonic fibroblasts (MEFs) expressing GFP, GFP-Sam68, or GFP-Sam68 (ΔN) proteins were mock- or γ-irradiated (IR) at 10 Gy for 5 min and whole cell lysates were derived and IB for indicated proteins, with β-actin as a loading control. (C) Sam68 KO MEFs expressing GFP, GFP-Sam68, or GFP-Sam68 (ΔN) proteins were stimulated with 10 μM of Camptothecin (CPT) for indicated periods, and cytosolic and nuclear fractions were derived and IB for indicated proteins. Caspase-3 (Casp3) and PARP1 served as loading controls and cytosolic and nuclear markers, respectively. (D) Indicated recombinant proteins were incubated in reaction buffer containing damaged DNA in the presence and absence of NAD+ and PARP1 inhibitor PJ-34. The reaction mixture was separated and IB for indicated proteins. Data are representative of at least three independent experiments.
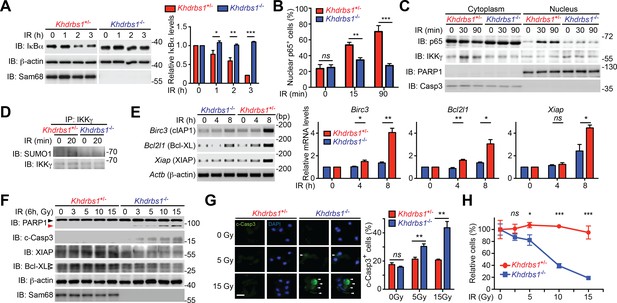
Sam68 deficiency attenuates NF-κB-mediated anti-apoptotic transcription in mouse colonic epithelial cells (CECs) under genotoxic stresses and sensitizes CECs to death.
(A) Whole cell lysates from primary Khdrbs1+/- and Khdrbs1-/- CECs treated with 10 Gy of γ-irradiation (IR) for indicated periods were immunoblotted (IB) for IκBα and Sam68, with β-actin as a loading control. Right, the IκBα levels, normalized to β-actin and untreated controls, were quantified from three independent experiments. (B) Isolated Khdrbs1+/- and Khdrbs1-/- CECs were treated with 10 Gy of IR for indicated periods, and fixed cells were stained for p65 and nuclei and subjected to immunofluorescence micrographs. Percentage of CECs (>100 cells from 5–8 random fields) with nuclear p65 staining was quantified. (C) Cytosolic and nuclear fractions derived from Khdrbs1+/- and Khdrbs1-/- CECs treated as in (B) were IB for indicated proteins. Cytosolic Caspase-3 (Casp3) and PARP1 served as loading controls and cytosolic and nuclear markers, respectively. (D) Whole cell lysates from Khdrbs1+/- and Khdrbs1-/- CECs stimulated with 10 Gy of IR for indicated periods, were IB for indicated proteins after immunoprecipitation (IP) with IKKγ antibody. (E) Total RNA was extracted from Khdrbs1+/- and Khdrbs1-/- CECs at indicated time points following IR (10 Gy) and mRNA profiles of Birc3, Bcl2l1, Xiap, and Actb were analyzed by semi-quantitative RT-PCR. Right, the relative expression levels of Birc3, Bcl2l1 and Xiap, normalized to Actb and untreated controls, were quantified from three independent experiments. (F) Khdrbs1+/- and Khdrbs1-/- CECs were γ-irradiated with indicated doses for 6 hr, and whole cell lysates were derived and IB for indicated proteins. c-Casp3, cleaved Caspase-3. The full-length and cleaved PARP1 are indicated by a black triangle and a red triangle, respectively; the two species of Bcl-XL proteins are labeled by open triangles. (G) Immunofluorescence micrographs of c-Casp3 in CECs treated as in (F), with nuclei counterstained by DAPI. Scale bar, 10 μm. Percentage of CECs (>100 cells from 5–8 random fields) with c-Casp3 staining was quantified (right). (H) Khdrbs1+/- and Khdrbs1-/- CECs were treated as in (F), and live cells following γ-irradiation were counted using a particle counter and normalized to the un-irradiated controls. Results in (A, B, E, G and H) are expressed as mean and s.e.m. ns, non-significant difference; *p<0.05; **p<0.01; ***p<0.001 by Student’s t tests. Data are representative of at least three independent experiments.
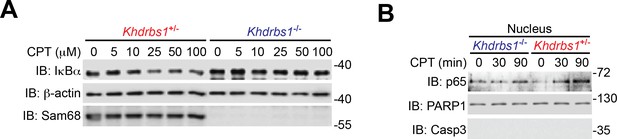
Sam68 deletion attenuates genotoxic stress-induced NF-κB signaling cascade in primary mouse cells.
(A) Whole cell lysates from isolated Khdrbs1+/- (Sam68 heterozygote) and Khdrbs1-/- (Sam68 knockout) colonic epithelial cells (CECs) treated with indicated concentrations of Camptothecin (CPT) for 2 hr were immunoblotted (IB) for IκBα and Sam68, with β-actin as a loading control. (B) Nuclear fractions derived from isolated Khdrbs1+/- and Khdrbs1-/- CECs stimulated with 25 μM of CPT for indicated periods were IB for indicated proteins.
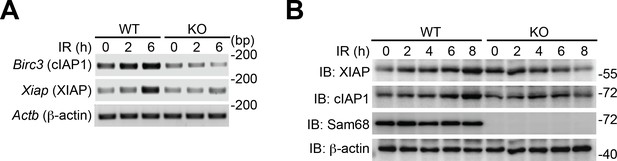
Sam68 deficiency attenuates DNA damage-triggered NF-κB-mediated expression of anti-apoptotic molecules.
(A) Wild-type (WT) and Sam68 knockout (KO) mouse embryonic fibroblasts (MEFs) were γ-irradiated (IR, 10 Gy) and total RNA was extracted at indicated time points. The mRNA profiles of Birc3, Xiap, and Actb were analyzed by semi-quantitative RT-PCR. (B) WT and Sam68 KO MEFs were γ-irradiated (IR, 10 Gy) for indicated periods and whole cell lysates derived and IB for indicated proteins, with β-actin as a loading control.
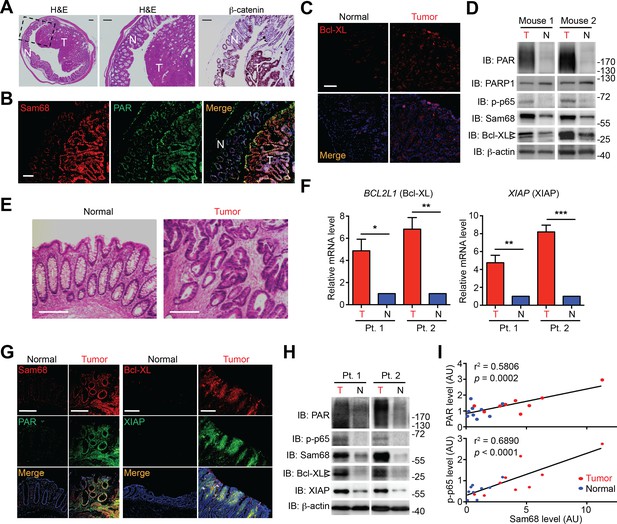
Sam68, PAR, and NF-κB-mediated anti-apoptotic transcription are elevated in mouse and human colon cancers.
(A and E) Hematoxylin and eosin (H&E) staining and β-catenin immunohistochemistry of colon sections from tumor-loaded Apcmin716/+ mice (A) and tissue sections of colon tumor or adjacent normal colon tissue from human cancer patients (E). Scale bars, 200 μm. N, normal tissue; T, tumor tissue. (B, C and G) Immunofluorescence micrographs of indicated proteins in colon sections from tumor-loaded Apcmin716/+ mice (B, C) or Normal and Tumor tissue derived from human colon cancer patients (G), with nuclei counterstained by DAPI. Scale bars, 100 μm. (D and H) Colonic epithelial cells were isolated from normal (N) or tumor (T) colon tissue from tumor-loaded Apcmin716/+ mice (D) or normal (N) and tumor (T) tissue derived from human colon cancer patients (Pt.) (H) and whole cell lysates were derived and immunoblotted for indicated proteins, with β-actin as a loading control. The two species of Bcl-XL proteins are labeled by open triangles. (F) Relative mRNA levels of BCL2L1 and XIAP, normalized to ACTB, from normal (N) and tumor (T) tissue derived from human colon cancer patients (Pt.). (I) Linear regression analysis of the levels of Sam68 protein versus PAR and phosphorylated p65 in CECs from normal (blue) and tumor (red) tissue derived from human colon cancer patients. AU, arbitrary unit. Results in (F) are expressed as mean and s.e.m. ns, non-significant difference; *p<0.05; **p<0.01; ***p<0.001 by Student’s t tests. Data in (A–H) are representative of at least three independent experiments.
-
Figure 5—source data 1
Surgical colorectal cancer (CRC) and polyp metadata.
Listed are the metadata of the surgical CRC and polyp from human patients, which have been used for this research. NA, not available.
- https://doi.org/10.7554/eLife.15018.017
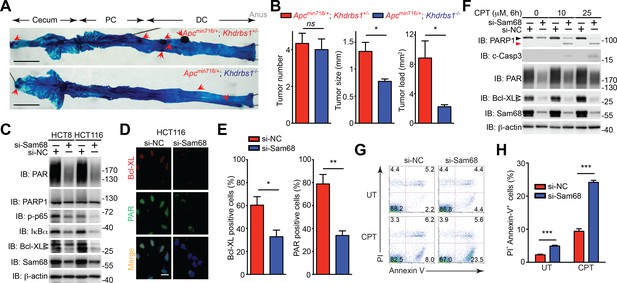
Sam68 plays a critical protective role for the survival of mouse and human colon cancers.
(A) Methylene blue (MB) staining of the colons (with cecum, proximal colon [PC], distal colon [DC], and anus indicated) derived from 3-month old Apcmin716/+; Khdrbs1+/- and Apcmin716/+; Khdrbs1-/- mice. Red arrows indicate colon tumors. Scale bar, 1 cm. (B) Quantification of tumor number, tumor size, and tumor load in the colons from Apcmin716/+; Khdrbs1+/- (n = 6) and Apcmin716/+; Khdrbs1-/- mice (n = 3) following MB staining. (C) HCT8 and HCT116 cells were transfected with nonspecific control (si-NC) or Sam68-specific (si-Sam68) small interference RNAs. 72 hr later, whole cell lysates were derived and immunoblotted (IB) for indicated proteins, with β-actin as a loading control. (D) Immunofluorescence micrographs of Bcl-XL and PARylated (PAR) proteins in the si-NC and si-Sam68 transfected HCT116 cells, with nuclei counterstained by DAPI. Scale bar, 20 μm. (E) Percentage of HCT116 cells (>100 cells from 5–8 random fields) with Bcl-XL and PAR staining was quantified. (F) HCT116 cells transfected with indicated siRNAs as in (C) were stimulated with indicated doses of Camptothecin (CPT) for 6 hr. Whole cell lysates were derived and IB for indicated proteins, with β-actin as a loading control. c-Casp3, cleaved Caspase-3. The full-length and cleaved PARP1 are indicated by a black triangle and a red triangle, respectively; the two species of Bcl-XL proteins are labeled by open triangles. (G) HCT8 cells transfected with indicated siRNAs as in (C) were left untreated (UT) or stimulated with 10 μM of CPT for 12 hr, followed by propidium iodide (PI)/Annexin V staining and flow cytometry analysis. (H) Percentages of apoptotic (PI- Annexin V+) HCT8 cells treated as in (G) were quantified. Results in (B, E, and H) are expressed as mean and s.e.m. ns, non-significant difference; *p<0.05; **p<0.01; ***p<0.001 by Student’s t tests. Data in (A and C–H) are representative of at least three independent experiments.
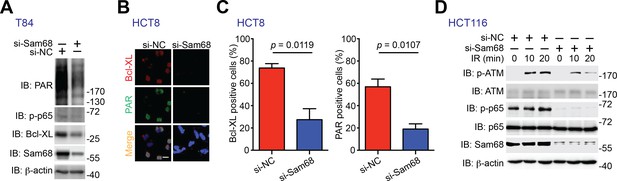
Sam68 knockdown and PARP inhibition attenuate PAR synthesis and PAR-dependent NF-κB transactivation in human colon cancer cell lines.
(A) T84 cells were transfected with nonspecific control (si-NC) or Sam68-specific (si-Sam68) small interference RNAs. 72 hr later, whole cell lysates were derived and immunoblotted (IB) for indicated proteins, with β-actin as a loading control. (B) Immunofluorescence micrographs of Bcl-XL and PARylated (PAR) proteins in the si-NC and si-Sam68 transfected HCT8 cells, with nuclei counterstained by DAPI. Scale bar, 20 μm. (C) Percentage of HCT8 cells (>100 cells from 5–8 random fields) with Bcl-XL and PAR staining was quantified. Results in (C) are expressed as mean and s.e.m. The p values are calculated by Student’s t tests. (D) HCT116 cells were transfected with si-NC or si-Sam68 small interference RNAs. 72 hr later, cells were subjected to 10 Gy of γ-irradiation (IR) and harvested at the indicated time periods post IR. Whole cell lysates were derived and IB for indicated proteins, with β-actin as a loading control. p-ATM, Ser1981 phosphorylated ATM; p-p65, Ser536 phosphorylated p65.
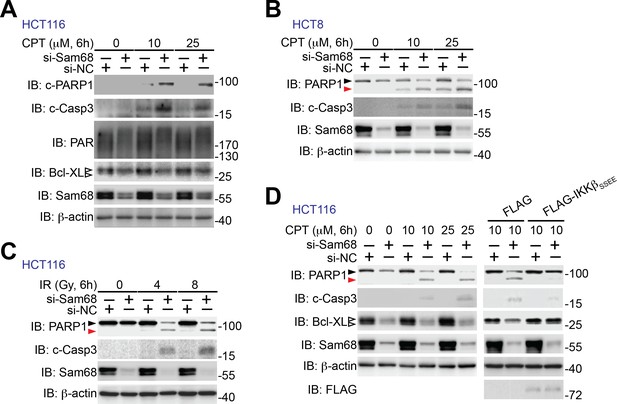
Sam68 knockdown sensitizes human colon cancer cells to genotoxic stress-induced apoptosis.
(A–C) HCT116 and HCT8 cells were transfected with nonspecific control (si-NC) or Sam68-specific (si-Sam68) small interference RNAs. 72 hr later, HCT116 (A) and HCT8 (B) cells were stimulated with indicated doses of Camptothecin (CPT) for 6 hr and HCT116 cells were γ-irradiated with indicated doses (C). Whole cell lysates were derived and immunoblotted (IB) for indicated proteins, with β-actin as a loading control. c-PARP1, cleaved PARP1; c-Casp3, cleaved Caspase-3. The two species of Bcl-XL proteins are labeled by open triangles (A). The full-length and cleaved PARP1 are indicated by a black triangle and a red triangle, respectively (B, C). (D) HCT116 cells expressing si-NC or si-Sam68 siRNA were transfected with either FLAG vehicle control or FLAG-IKKβ (SSEE) plasmid. 18 hr later, the cells were stimulated with 10 μM of CPT for 6 hr, and whole cell lysates were derived and IB for indicated proteins, with β-actin as a loading control. c-Casp3, cleaved Caspase-3. The full-length and cleaved PARP1 are indicated by a black triangle and a red triangle, respectively; the two species of Bcl-XL proteins are labeled by open triangles. The left of this panel was duplicated from the blots in Figure 6F to illustrate the rescuing effect of ectopic expression of IKKβ (SSEE).
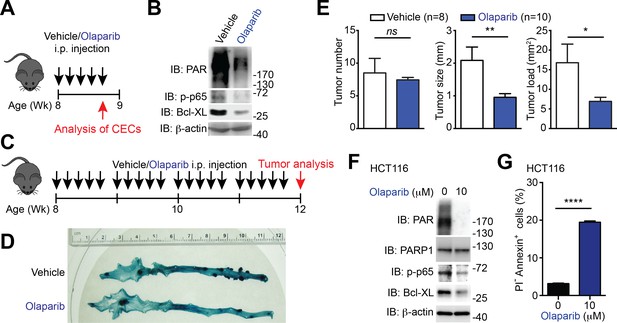
PARP1 inhibition reduces colon tumor development in mice and sensitizes human colon cancer cells to undergo apoptosis.
(A) PARP1 inhibition in Apcmin716/+ mice in vivo. 8-week-old Apcmin716/+ mice were intraperitoneally injected with vehicle control or Olaparib (50 mg/kg) once daily for 5 days, followed by euthanization and further analysis. (B) Colon epithelial cells (CECs) were isolated from vehicle- or Olaparib-treated Apcmin716/+ mice, treated as in (A), and whole cell lysates were derived and immunoblotted (IB) for indicated proteins, with β-actin as a loading control. (C) A schematic of the experimental timeline for the impact of PARP1 inhibition on colon tumor development in vivo in Apcmin716/+ mice. 8-week-old Apcmin716/+ mice were intraperitoneally injected vehicle control or Olaparib (50 mg/kg, once daily for 5 days × 4 weeks). Mice were euthanized to analyze tumor development in the colon. (D) Methylene blue (MB) staining of the colons derived from 12-week old Apcmin716/+ mice, post 4-week vehicle control or Olaparib treatment, as illustrated in (C). (E) Quantification of tumor number, tumor size, and tumor load in the colons from Apcmin716/+ mice treated with vehicle control (n = 8) and Olaparib (n = 10), following MB staining. (F) HCT116 cells were treated with indicated concentration of Olaparib for 72 hr. Whole cell lysates were derived and IB for indicated proteins, with β-actin as a loading control. (G) HCT116 cells were treated with Olaparib as in (F), and subjected to flow cytometry analysis of propidium iodide (PI)/Annexin V staining. Percentages of apoptotic (PI- Annexin V+) cells as indicated were quantified. Results in (E and G) are expressed as mean and s.e.m. ns, non-significant difference; *p<0.05; **p<0.01; ****p<0.0001 by Student’s t tests. Data in (B, D, F, and G) are representative of at least three independent experiments.
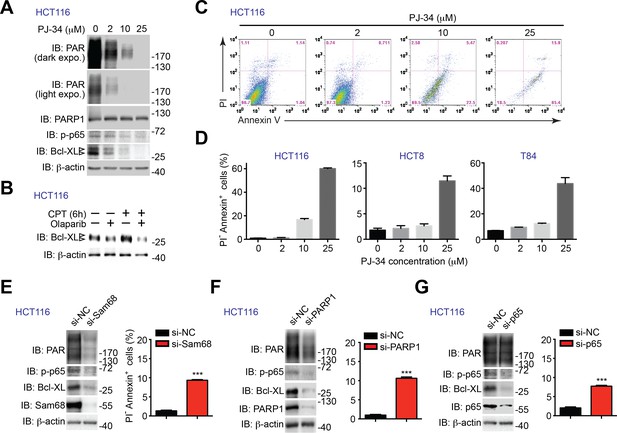
PARP1 inhibition and down-regulation of PARP1 and NF-κB triggers human cancer cells undergo apoptosis.
(A) HCT116 cells were treated with indicated concentration of PJ-34 for 72 hr. Whole cell lysates were derived and immunoblotted (IB) for indicated proteins, with β-actin as a loading control. The two species of Bcl-XL proteins are labeled by open triangles. (B) HCT116 cells, pretreated with 10 μM of Olaparib or vehicle control for 1 hr, were stimulated with (+) or without (−) 10 μM of Camptothecin (CPT) for 6 hr. Whole cell lysates were derived and IB for Bcl-XL, with β-actin as a loading control. The two species of Bcl-XL proteins are labeled by open triangles. (C) HCT116 cells treated as in (A), were stained by propidium iodide (PI)/Annexin V, followed by flow cytometry analysis. (D) HCT8, HCT116, and T84 cells were treated with PJ-34 as in (A), and subjected to flow cytometry analysis of PI/Annexin V staining. Percentages of apoptotic (PI- Annexin V+) cells as indicated were quantified from three representative experiments. (E, F, and G) HCT116 were transfected with nonspecific control (si-NC), Sam68-specific (si-Sam68) (E), PARP1-specific (si-PARP1) (F), or p65-specific (si-p65) (G) small interference RNAs. 72 hr later, whole cell lysates were derived and IB for indicated proteins, with β-actin as a loading control. Right, HCT116 cells transfected as in (E, F,and G) were stained by PI/Annexin V, followed by flow cytometry analysis. Percentages of apoptotic (PI- Annexin V+) cells were quantified from three representative experiments. Results in (D–G) are expressed as mean and s.e.m. , ***p<0.001 by Student’s t tests.
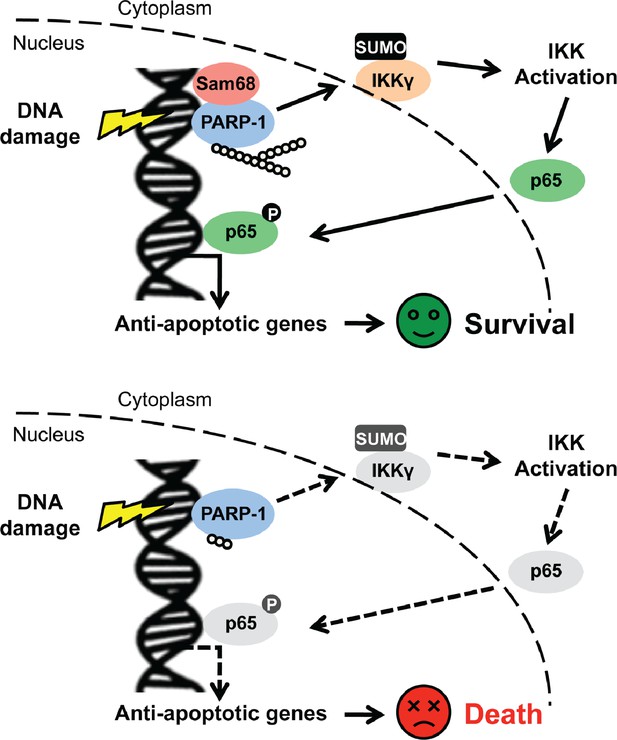
Schematic model representation of Sam68 functioning as an early signaling molecule in genotoxic stress-initiated NF-κB signaling pathway.
https://doi.org/10.7554/eLife.15018.023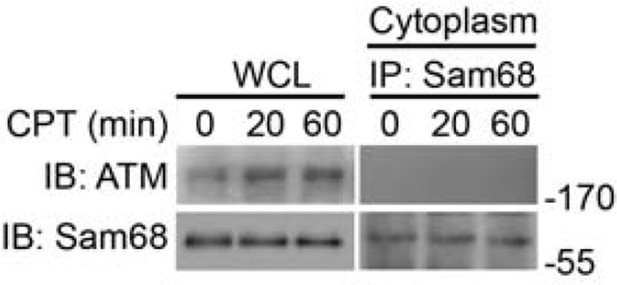
Whole cell lysates (WCL) from wild-type MEFs stimulated with 10 μM of CPT for indicated periods were immunoblotted (IB) directly or after subcellular fractionation and immunoprecipitation (IP) with Sam68 antibody for indicated proteins.
https://doi.org/10.7554/eLife.15018.024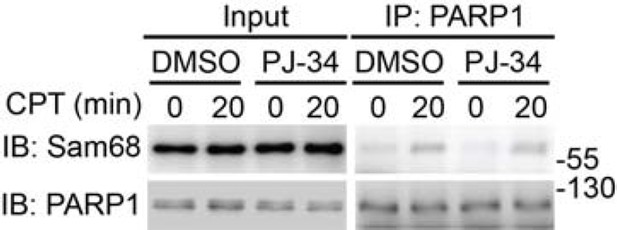
Wild-type MEFs, pretreated with DMSO or PJ-34 (10 μM) for 1h, were stimulated with 10 μM of CPT for indicated periods.
Whole cell lysates (Input) were immunoblotted (IB) directly or after immunoprecipitation (IP) with PARP1 antibody for the indicated proteins.
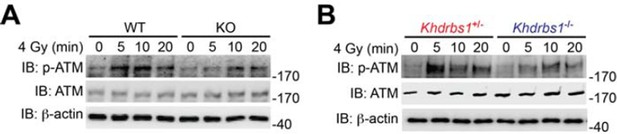
(A) Wild-type (WT) and Sam68 knockout (KO) mouse embryonic fibroblasts (MEFs) were γ-irradiated (IR) at 4 Gy and whole cell lysates were derived at indicated time points post IR and immunoblotted (IB) for the indicated proteins, with β-actin as a loading control. (B) Primary thymocytes isolated from Khdrbs1+/- and Khdrbs1-/- mice were IR at 4 Gy and whole cell lysates were derived at indicated time points and IB for the indicated proteins, with β-actin as a loading control. p-ATM, Ser1981 phosphorylated ATM.





