Dynamic metabolic exchange governs a marine algal-bacterial interaction
Figures
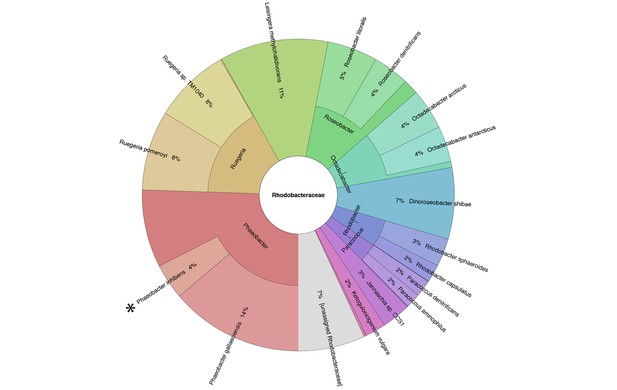
Metagenomic analysis of Roseobacters associated with E. huxleyi blooms reveals co-occurrence of P. inhibens.
Two E. huxleyi blooms were sampled in the Gulf of Maine during the summer of 2015 and metagenomic analysis of the bacterial population was performed (see Materials and methods). Shown is the relative abundance of members of the Rhodobacteraceae family, which accounted for 6% of bacteria. The same members of the Rhodobacteraceae family were detected in both blooms and their abundance changed ±2% between replicates and between the two blooms. P. inhibens was present in both blooms and is indicated by an asterisk. Shown are the results for the July 2015 bloom (see Materials and methods).
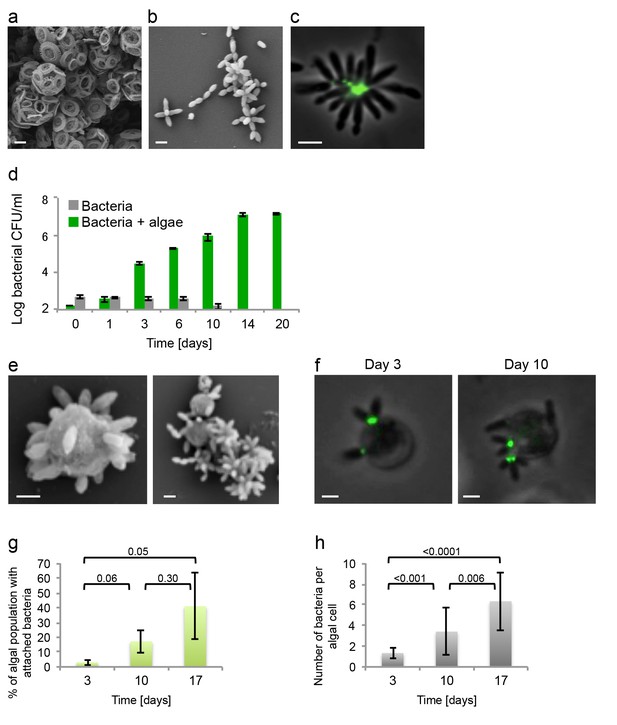
Algal-bacterial co-cultures.
(a) Scanning electron microscopy (SEM) image of E. huxleyi (CCMP3266) pure algal culture. (b) SEM image of P. inhibens (DMS17395) pure bacterial culture. (c) Overlay image of a pure culture of P. inhibens bacteria (phase contrast microscopy, grey) stained with a fluorescent lectin (Alexa Fluor 488 conjugated lectin, green). (d) Bacteria grown in L1-Si medium in the absence (grey bars) and presence (green bars) of algae over 20 days. Error bars represent the standard deviation of two biological replicates. (e) SEM image of cells from an algal-bacterial co-culture. (f) Phase contrast microscopy imaging of live co-culture samples (grey) overlaid with images of the fluorescent lectin (Alexa Fluor 488 conjugated lectin, green) showing increasing numbers of bacteria attaching onto algal cells over time. (g) Quantification of algal cells with attached bacteria as a function of time, n > 300. Error bars represent the standard deviation between the multiple examined fields. (h) Quantification of the number of attached bacteria per algal cell as a function of time, n > 300. Error bars represent the standard deviation between the multiple examined fields. All scale bars in the figure correspond to 1 µm. Statistical significance was calculated using a Student’s T-test and p values are presented above datasets.
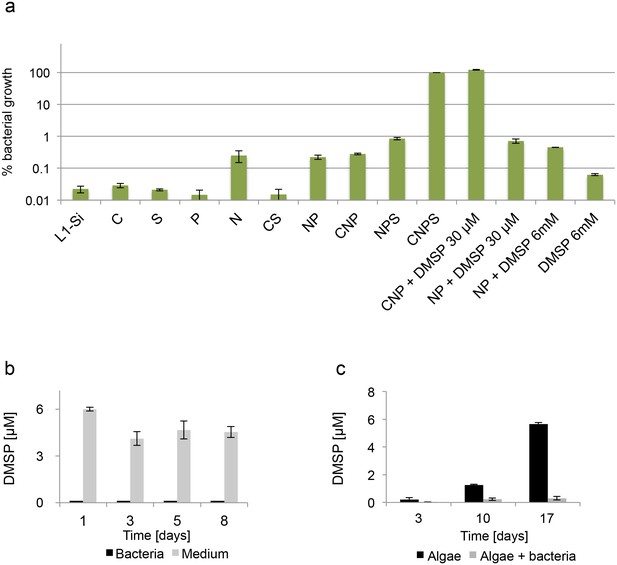
Bacteria require essential nutrients to grow in L1-Si.
(a) Bacterial growth in L1-Si medium supplemented with various essential nutrients (C-glucose, N-nitrogen, P-phosphorus, S-sulfur) was monitored over eight days (see Materials and methods). Presented are the maximal growth values that were obtained after three days of incubation. The initial bacterial inoculum was 1×105 CFU/ ml. Growth in CNPS reached 5×108 and was normalized to 100%. (b) Bacteria consume externally added DMSP. (c) DMSP production by E. huxleyi in pure culture (black bars) and in co-culture (grey bars). Error bars represent the standard deviation between two biological replicates.
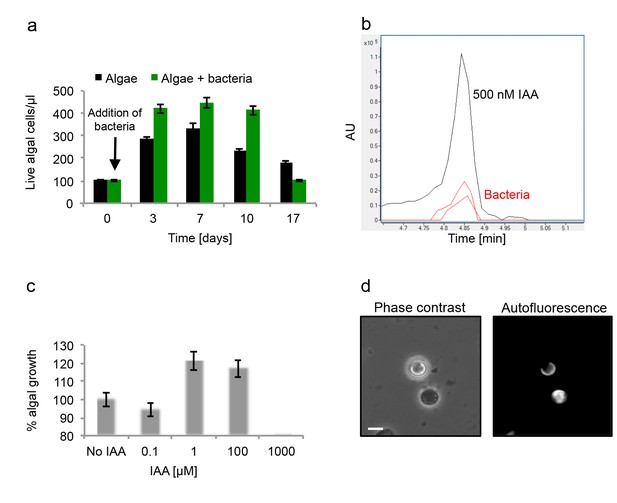
Algal growth in the presence of bacteria.
(a) Algal growth was monitored over 17 days in the presence of bacteria (green bars) and in pure culture (black bars). (b) Indole-3-acetic acid (IAA) production was observed in pure bacterial cultures grown in L1-Si supplemented with essential nutrients. Shown is an LC-MS extracted ion chromatogram (EIC, m/z 176.0706 ± 10 ppm) of an IAA standard of 500 nM (black) and two biological replicates (red) (see Materials and methods). AU=Arbitrary Units. (c) Algal growth was examined upon addition of the auxin IAA. Percent algal growth is relative to a culture with no IAA added. Note that at a concentration of 1000 μM IAA, cell numbers dropped to less than 10%. (d) Following treatment with 1 mM IAA, the autofluorescence signal of dead algal cells (the lower cell in this image) appears similar to the signal observed in bacterially induced death (compare with Figure 6l), indicative of chloroplast deformation but partially intact cell membrane. Scale bar corresponds to 4 μm. Error bars in a and c represent the standard deviation between two biological replicates.
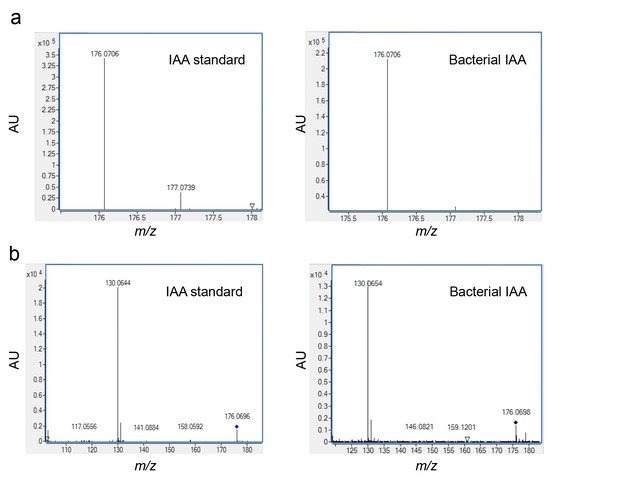
Validating the detection of bacterially produced IAA.
(a) The accurate mass of the bacterial IAA was determined using High Resolution LC-MS. Identical m/z values were obtained for an IAA standard and bacterially produced IAA. (b) Both IAA standard and bacterial IAA exhibit characteristic MS/MS fragmentation patterns, such as the shown fragment m/z 130.
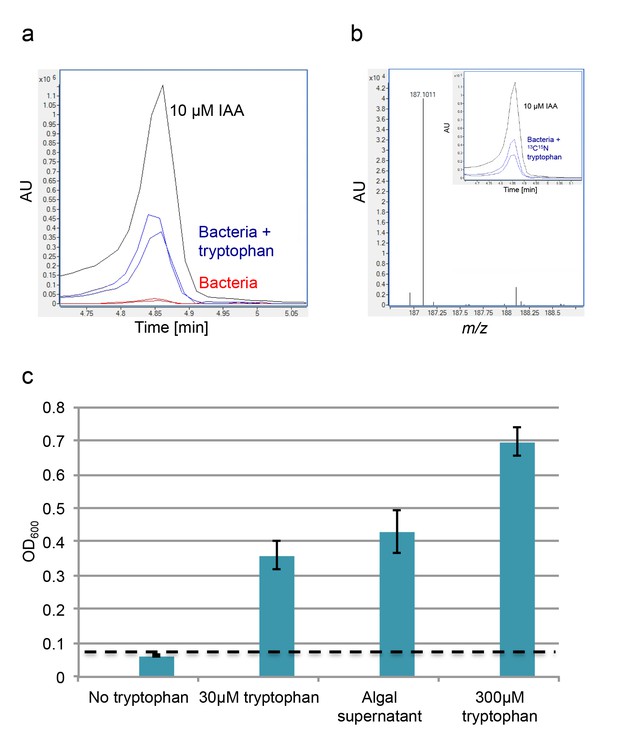
Exogenous tryptophan promotes bacterial IAA biosynthesis.
(a) Addition of 0.1 mM tryptophan to P. inhibens cultures results in approximately 25-fold increase in produced IAA. Shown is an LC-MS extracted ion chromatogram (EIC, m/z 176.0706 ± 10 ppm) of an IAA standard of 10 μM (black), IAA detected in bacterial cultures supplemented with tryptophan (blue), and IAA detected in untreated bacterial cultures (red). (b) The addition of isotopically labeled tryptophan (13C and 15N) leads to bacterial production of IAA with full isotopic incorporation, indicated by m/z 187.0011. Inset shows the LC-MS chromatogram of an IAA standard (black) (EIC, m/z 176.0706 ± 10 ppm) and the labeled IAA detected in two biological replicates (blue) (EIC, m/z 187.0011 ± 10 ppm). (c) Cell density (OD600) after 16 hr at 30°C of a tryptophan auxotroph E. coli strain grown in known concentrations of tryptophan and in algal supernatant (see Materials and methods). Dashed line indicates density of the initial inoculum. Error bars represent the standard deviation between four biological replicates.
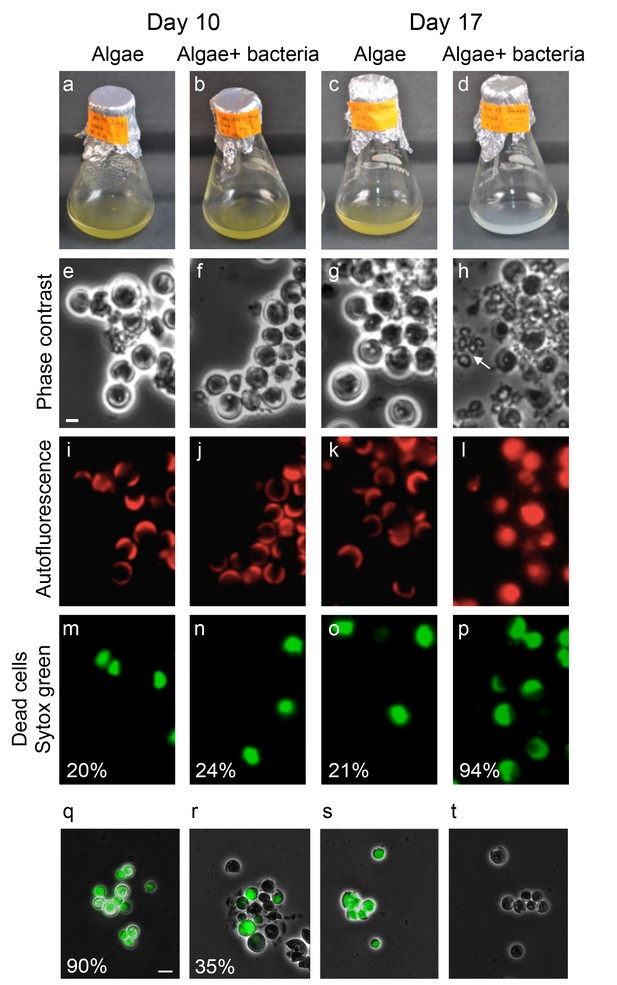
Bacteria induce a unique algal death in aging co-cultures.
(a–d) Images of cultures demonstrating the change in the culture color over time. (e–h) Phase contrast microscopy images. Arrow points to shed coccoliths. (i–l) Fluorescent images of chlorophyll and accessory pigments autofluorescence. (m–p) Fluorescent images of dead cells stained with Sytox green. Percentages indicate the number of dead cells counted in each population. For each value n > 300 and the standard deviation between several analyzed fields was up to 20% of the indicated value. (q–t) Overlay of phase contrast microscopy images (grey) with fluorescent images of TUNEL assay (green) of cultures at day 20 (see Materials and methods). (q) Co-culture, (r) Axenic algal culture, (s) Positive control, cells were pretreated with DNase I, (t) Negative control, the terminal deoxynucleotidyl transferase enzyme (TdT) was replaced with distilled water. Percentages indicate the number of positively stained cells counted in each population. For each value n > 300 and the standard deviation between several analyzed fields was up to 25% of the indicated value. Scale bar corresponds to 1 µm in e–p and 4 µm in q–t.
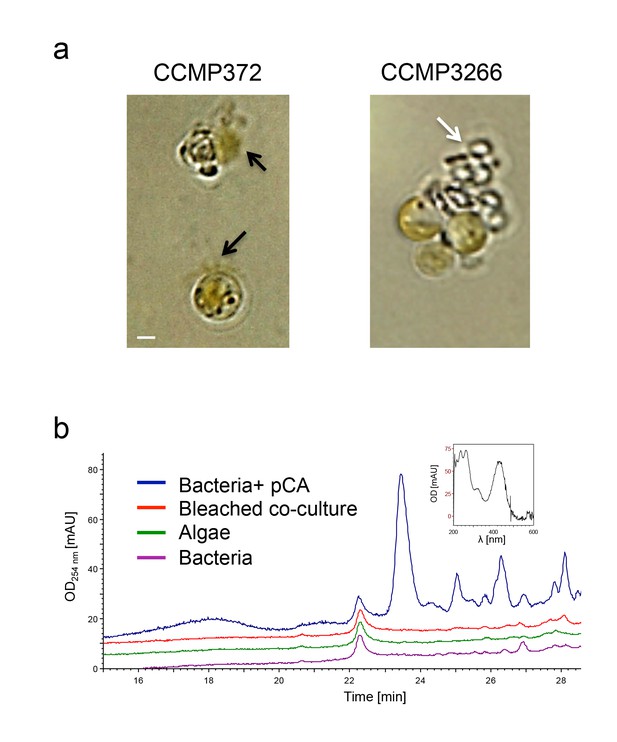
Roseobacticide-mediated algal lysis is strain specific.
(a) Roseobacticides were introduced into 1 ml of algal cultures of E. huxleyi strains CCMP372 and CCMP3266. After 12 hr, cell lysis accompanied by chloroplast discharge was evident only in strain CCMP372 (black arrows). White arrow points to coccoliths in the image of the calcifying strain CCMP3266. Control cultures treated with equivalent volumes of solvent (methanol) or medium, did not exhibit any change (not shown). Scale bar corresponds to 1 µm. (b) Roseobacticides were extracted from various cultures (see Materials and methods). Roseobacticides were detected in bacterial culture grown in the presence of para-coumaric acid (‘Bacteria + pCA’, blue). Inset showing the characteristic absorbance peak of Roseobacticides at 430 nm (Seyedsayamdost et al., 2011). No Roseobacticides were detected in a bleached co-culture (red) or in control pure cultures of algae (green) or bacteria (purple) grown without addition of pCA.
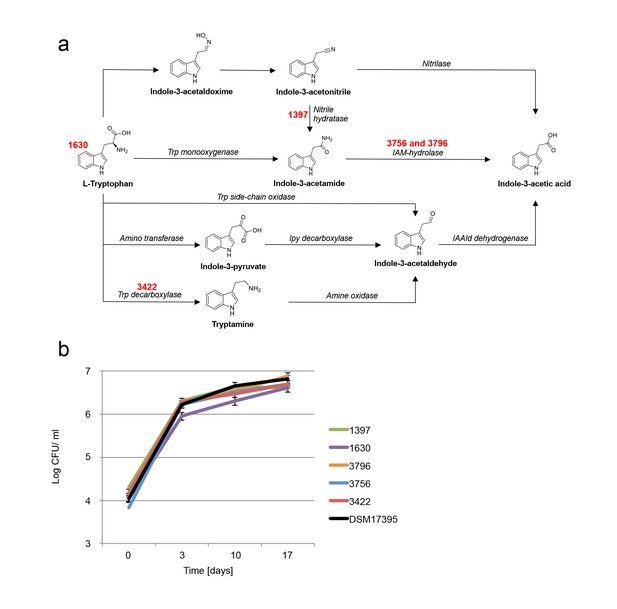
Examination of bacterial mutants.
(a) Mapping the different mutants onto the various bacterial IAA biosynthetic pathways. Mutant strain numbers (see Table 2) are indicated in red next to the pathway that was mutated. Known bacterial enzymes appear in italic. Trp - tryptophan, IAM - indole-3-acetamide, Ipy - indole-3-pyruvate, IAAId - indole-3-acetaldehyde. Image modified from Spaepen et al. (2007). (b) Mutants in various biosynthetic pathways of indole-3-acetic acid (IAA) production were grown in co-culture with algae over a period of 17 days. As can be seen, all mutants exhibited growth dynamics similar to the wild type DSM 17395 strain. Of note, mutant 1630 that is a tryptophan auxotroph (see Table 2) could not grow in pure culture in the absence of tryptophan but was able to grow in co-culture. Error bars represent the standard deviation of two biological replicates.
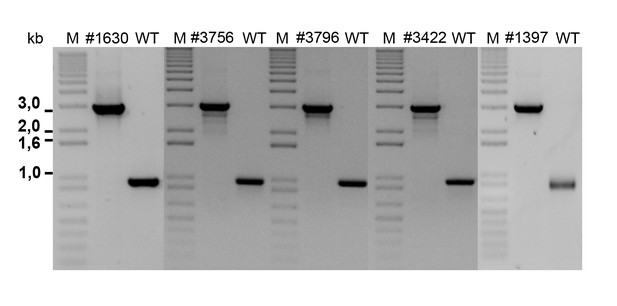
Agarose gel of PCR amplifications confirming the transposon insertion site of select sequenced mutants in P. inhibens.
Specific primers were designed to amplify the transposon insertion site (Table 3). As can be seen, amplicons from mutants result in a 2 kb increase in size due to the insertion of a transposon harboring the kanamycin resistance cassette. M- marker, WT- wild type DSM 17395.

In bacteria, exogenous tryptophan serves as both a precursor and a cue.
(a) Images of co-cultures and algal cultures at day 10. Upon inoculation of bacteria, 0.1 mM tryptophan (trp) was added. Seen is a co-culture that bleached a week earlier (middle). An algal culture treated with the same concentration of tryptophan did not display bleaching (right). (b–c) Phase contrast microscopy imaging of co-cultures at day 10 with (+trp) and without (−trp) addition of 0.1 mM tryptophan. Scale bar corresponds to 3 μm. Note that in the tryptophan treated co-culture (+trp) in (c) each algal cell has attached bacteria and the bacterial coverage is so dense that several covered algal cells cannot be seen.
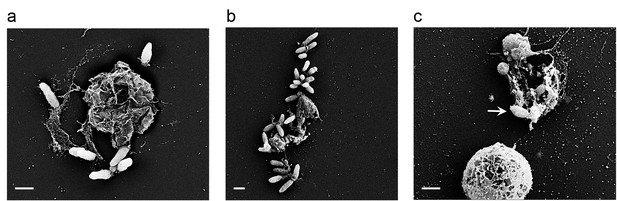
Bacteria attached to remains of dead algal cells.
(a–c) SEM images of co-cultures at day 17 of incubation reveal bacteria that are attached to algal debris. The vast majority of imaged algal cells were intact (see for example the lower cell in panel c) indicating that algal debris were not generated as a result of sample preparation. Arrow pointing at a bacterial cell attached to algal remains that are adjacent to an intact algal cell. Scale bar corresponds to 1 μm.
Videos
Live imaging of the algal bleaching in co-culture.
https://doi.org/10.7554/eLife.17473.010Tables
Expression data ratios between algae in co-culture and in axenic culture. Select genes involved in stress response and programmed cell death show up-regulation over time.
| Functional group | Gene annotation | Target transcript | Day 3 | Day 10 | Day 17 |
|---|---|---|---|---|---|
Oxidative stress | putative L-ascorbate peroxidase | XM_005793355 | 1.36 | 3.19 | 6.81 |
Programmed cell death | putative programmed cell death protein (PDCD2) | XM_005768970 | 1.31 | 2.13 | 5.29 |
Metacaspases | putative metacaspase protein with Ca-binding EF hand domain | XM_005784588 | 1.31 | 3.30 | 4.54 |
Metacaspases | putative metacaspase protein | XM_005763016 | 1.05 | 2.32 | 4.39 |
Oxidative stress | ascorbate oxidase (AO) | XM_005775302 | 1.69 | 2.82 | 4.09 |
Oxidative stress | putative glutathione-S-transferase | XM_005761417 | 1.31 | 2.20 | 4.07 |
Metacaspases | putative metacaspase protein | XM_005791576 | 1.99 | 2.65 | 4.03 |
Programmed cell death | putative death-specific protein with Ca binding EF hand domain | XM_005778875 | 1.48 | 2.40 | 3.88 |
Programmed cell death | putative programmed cell death protein (PDCD2) | XM_005790372 | 1.21 | 2.12 | 3.84 |
Metacaspases | putative metacaspase protein | XM_005773908 | 1.46 | 2.28 | 3.83 |
Oxidative stress | glutathione synthetase (GSHS3) | XM_005760150 | 1.32 | 2.14 | 3.56 |
Oxidative stress | putative L-ascorbate peroxidase | XM_005784352 | 1.17 | 2.19 | 3.33 |
Programmed cell death | putative death-specific protein with Ca binding EF hand domain | XM_005773034 | 1.41 | 2.00 | 3.22 |
Metacaspases | putative metacaspase protein | XM_005759676 | 1.17 | 1.90 | 3.06 |
Oxidative stress | thioredoxin | XM_005761968 | 1.03 | 2.01 | 2.75 |
P. inhibens mutant strains used in the current study.
| Strain | Genotype | Hypothetical function | IAA production in CNPS [nM] | IAA production in CNPS + trp [nM] |
|---|---|---|---|---|
| DSM 17395 | Wild type | 0.4 | 10.8 | |
| 3756 | PGA1_c11890:: Tn5 (KanR) | Indoleacetamide hydrolase | Yes | Yes |
| 3796 | PGA1_c11890:: Tn5 (KanR) | Indoleacetamide hydrolase | Yes | Yes |
| 1630 | PGA1_c16910:: Tn5 (KanR) | Indole-3-glycerol phosphate synthase | NA* | Yes |
| 1397 | PGA1_c23870:: Tn5 (KanR) | Nitrile hydratase subunit beta | Yes | Yes |
| 3422 | PGA1_c31390:: Tn5 (KanR) | Aromatic-L-amino-acid decarboxylase | Yes | Yes |
-
*NA - Not available since this mutant does not grow in these conditions without the addition of tryptophan
Chlorophyll a measurements in algal cultures. Chlorophyll a was measured spectroscopically in pure and co-cultures over time (see Materials and methods).
Sample ID | Chlorophyll a per cell [pg] |
|---|---|
Algae day 3 | 5.96×10−3 |
Co-culture day 3 | 6.18×10−3 |
Algae day 10 | 1.43×10−2 |
Co-culture day 10 | 1.67×10−2 |
Algae day 17 | 1.50×10−2 |
Co-culture day 17 | 6.55×10−3 |
Additional files
-
Supplementary file 1
Expression data ratios between algae in co-culture and in axenic culture.
- https://doi.org/10.7554/eLife.17473.019
-
Supplementary file 2
Primer sequences for validation of transposon insertion sites.
- https://doi.org/10.7554/eLife.17473.020
-
Supplementary file 3
Gene expression data.
- https://doi.org/10.7554/eLife.17473.021





