Fine-tuning of Notch signaling sets the boundary of the organ of Corti and establishes sensory cell fates
Figures
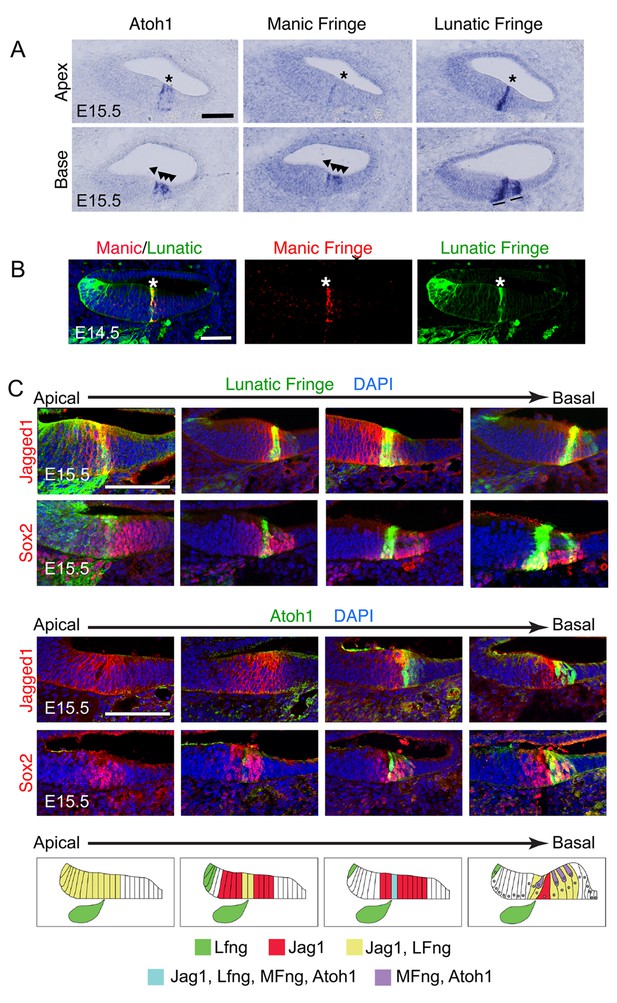
Dynamic expression of Lfng, Mfng and Jag1 during the onset of cochlear hair cell differentiation.
(A) Serial sections from the basal and apical turns of an E15.5 cochlea processed for in situ hybridization for Atoh1, Mfng and Lfng. At the apex, where hair cell differentiation is just commencing, Lfng and Mfng co-localize to the first Atoh1-expressing cells at the boundary of the developing organ of Corti (asterisks). In the more mature basal region, Mfng is co-expressed with Atoh1 in immature hair cells (arrowheads), while Lfng is restricted to supporting cells (black lines). A more complete series of sections is shown in Figure 1—figure supplement 1 (B) Mfng and Lfng co-localize in the region of differentiating hair cells (asterisk). Fluorescent in situ hybridization for Mfng mRNA (red) was performed on cochlear sections from Lfng-GFP BAC transgenic mice, immunostained with antibodies to GFP (green). (C) Expression of Jag1 protein and Lfng in relationship to differentiating hair cells. Sections spanning the apical-basal axis of the E15.5 cochlea were taken from Lfng-GFP BAC transgenic mice and Atoh1GFP/GFP knock-in mice. In each case, sections were stained with antibodies to GFP and to either Jag1 or Sox2 to mark prosensory cells and cells in Kölliker’s organ. The dynamic expression pattern is summarized in (D) – Jag1 and Lfng are expressed in Kölliker’s organ in the apex of the cochlea, then become restricted to supporting cells in the base. A stripe of Lfng and Mfng coincides with the first differentiating hair cells at the border of Kölliker’s organ. After hair cell differentiation initiates, Atoh1 and Mfng are restricted to hair cells. The position of innervation from the Lfng-expressing spiral ganglion afferents (green ganglion) at the site of the first inner hair cells is indicated in each schematic panel.
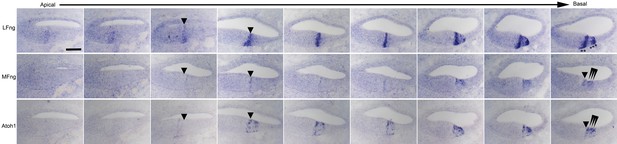
Detailed analysis of dynamic expression changes in Lfng, Mfng and Atoh1 expression along the apical-basal axis of the E15.5 cochlear duct.
Nine sets of three serial sections processed for in situ hybridization with ribroprobes for Lfng, Mfng and Atoh1 are shown along the length of the cochlear duct. The first localization of LFng and Mfng expression to the boundary of the prosensory domain and Kölliker’s organ is shown with an arrowhead. In the basal-most set of sections, differentiating Atoh1+, Mfng+ hair cells are shown with arrowheads, while Lfng+ supporting cells are shown with asterisks. Scale bar = 50 µm.
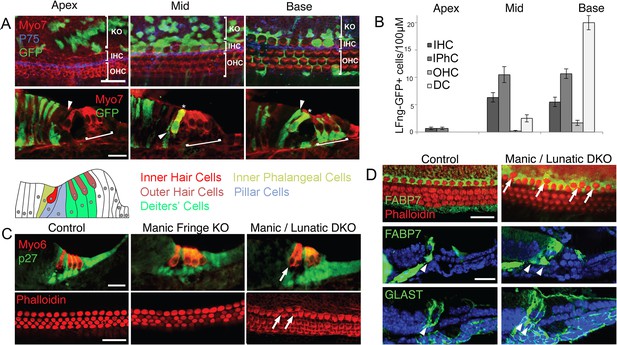
Fate mapping of Lfng progenitors and characterization of Lfng;Mfng mutant mice.
(A) Fate mapping with Lfng-CreEr mice shows that inner hair cells and inner phalangeal cells derive from of Lfng-expressing progenitors. Lfng-CreER mice were crossed with Ai3 Cre reporter mice and tamoxifen was administered at E14.5. Mice were allowed to develop until E18.5 and then apical, mid-turn and basal regions of the cochlea examined in whole mount and sections. In the apical, most immature region of the cochlea, no organ of Corti cells are labeled; however robust labeling is seen in cells of Kölliker’s organ. In the middle turn, both inner hair cells and their associated inner phalangeal cells are labeled, but no other cells in the organ of Corti are labeled. In the basal, most mature region of the cochlea, Deiters’ cells and pillar cells are strongly labeled in addition to the inner hair cell region. Occasional outer hair cells are also labeled. (B) Quantitation of GFP-labeled cells in the organ of Corti along the apical-basal axis. In the apex, small numbers of inner hair cells (IHC) and inner phalangeal cells (IPhC) are labeled with GFP. In mid-turn regions, many more of these cells are labeled, together with a small number of outer hair cells (OHC) and Deiters’ cells (DC). Deiters’ cells constitute the majority of labeled cells in basal regions of the cochlea. (C): Duplication of inner hair cells and their associated supporting cells in Lfng/Mfng double mutants. Sections and whole mount preparations of P0 control, Mfng−/− and Mfng−/−;Lfng−/− mutant mice. Sections show immunostaining for hair cells (Myosin6; red) and supporting cells (p27kip1; green), while whole mount preparations reveal hair cell actin with fluorescently labeled phalloidin (red). Mfng−/−;Lfng−/− mutant cochleas have regions containing ectopic inner hair cells (arrows). This phenotype is not observed in Mfng−/− embryos, (or Lfng−/− embryos; not shown, Zhang et al., 2000). (D) Duplication of inner hair cells in Mfng−/−;Lfng−/− mutant cochleas is accompanied by a duplication of the surrounding inner phalangeal cells. Whole mount preparations of control and double mutant cochleas are stained with fluorescently-labeled phalloidin (red) and antibodies to FABP7 (green) to label inner phalangeal cells. Sections of control and double mutant cochleas are stained with antibodies to either FABP7 or GLAST to reveal inner phalangeal cells. The duplicated inner phalangeal cell region is indicated with arrows.

Lineage tracing with LFng-CreER transgenic mice recapitulates the dynamic pattern of Lfng expression.
Lfng-CreER mice were mated with Ai3 Cre reporter females and a single dose of tamoxifen was administered at E14.5. The mice were sacrificed at E18.5 and stained with antibodies for GFP (green) and the pillar cell marker p75 (red). The entire stained cochlea is shown in whole mount, together with higher power images taken of apical, apical-middle and basal regions of the cochlea. In the apex, GFP-labeled cells are limited to Kölliker’s organ (yellow lines). In apical-middle turn regions, GFP labeling can now be observed in inner hair cells and inner phalangeal cells (white arrowheads). In the base of the cochlea, GFP labeling now extends into the rest of the organ of Corti where outer hair cells reside, labeling mostly Deiters’ cells (light blue brackets). We also illustrate a ‘transition zone’, where the GFP labeling begins to appear in the outer hair cell region (light blue brackets).
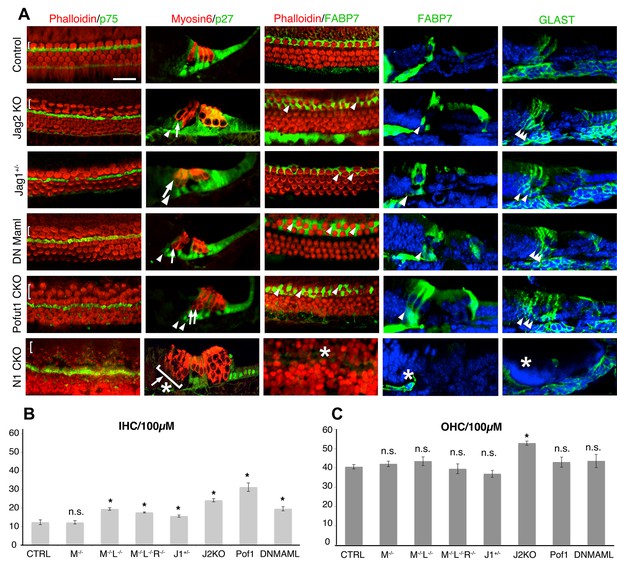
Duplication of inner hair cells and inner phalangeal cells in hypomorphic Notch loss-of-function alleles.
(A) Jag2−/− mutants; Jag1−/+heterozygotes and conditional Pofut1 and dnMAML1 mutants all display regions of duplicated inner hair cells and inner phalangeal cells. The inner hair cell region is shown on P0 cochlear whole mount preparations stained with phalloidin (hair cells; red) and either p75 (pillar cells; green) or FABP7 (inner phalangeal cells; green). Sections show all hair cells and supporting cells (Myosin6; red and p27kip1; green) or just inner phalangeal cells (FABP7 or GLAST; green). Ectopic inner phalangeal cells are shown with white arrows. White asterisks show absent supporting cells. (B) Inner hair cell numbers are significantly increased in Mfng−/−;Lfng−/− mutants, Mfng−/−;Lfng−/−; Rfng−/−mutants, Pofut1 and dnMAML1 conditional mutants and Jag1+/− and Jag2−/− mutants, but not Mfng-/- or control cochleas. (C) Outer hair cells numbers are only significantly increased in Jag2−/− mutants. In each case, bars represent the mean number of inner or outer hair cells per 100 µm (p<0.05; Student two-tailed t test). M: Mfng; L: Lfng, R: Rfng; Pof1: Pofut1; DNMAML: dnMAML1; J1: Jag1; J2: Jag2.
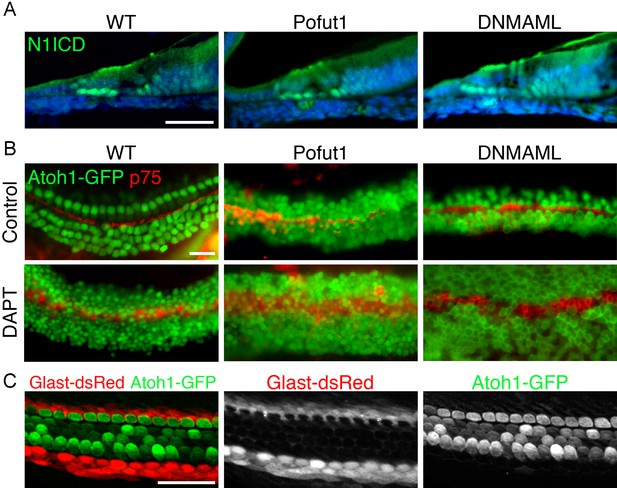
Additional characterization of Pofut1, dnMAML1 and GLAST-DsRed mice.
(A, B) The Pofut1 and dnMAML1 conditional alleles do not give a complete Notch loss-of-function. (A) Sections of neonatal wild type, Pofut1 and dnMAML1 conditional mutant cochleas processed with antibodies to the Notch1 intracellular domain (N1ICD; green). Staining can still be observed in supporting cells. (B) Treatment of cultured neonatal cochleas from wild type, Pofut1 and dnMAML1 conditional mutant mice in the presence of DAPT increases the numbers of supernumerary hair cells compared to untreated controls, again suggesting that neither allele represents complete Notch loss of function in the cochlea. (C) GLAST-DsRed mice show labeling of the inner phalangeal cell region. Surface preparations of cochleas from neonatal GLAST-DsRed;Atoh1GFP/GFP mice show strong DsRed labeling in the inner phalangeal cell region adjacent to GFP-expressing inner hair cells. Scale bar = 50 µm (A,B) and 20 µm (C).
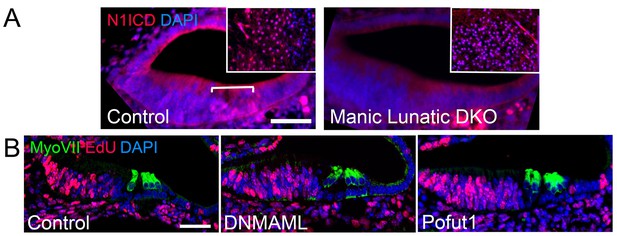
Notch signaling and EdU incorporation in Notch loss-of-function mutants.
(A) Notch signaling is reduced in Lfng;Mfng double mutants. Sections through the cochlear duct of E15.5 wild type and Lfng;Mfng double mutant are shown stained for the Notch1 intracellular domain (N1ICD). The insets show N1ICD staining in the hindbrain taken from the same section containing the cochlear duct as a positive control. In Lfng;Mfng double mutants, N1ICD staining is greatly reduced in the cochlea, but not the hindbrain. (B) Edu incorporation shows no significant increase in labeling of inner hair cells or inner phalangeal cells following a reduction in Notch signaling. We administered EdU to pregnant female mice three times a day between E14.5 and E17.5 and collected embryos for analysis at E18.5. We observed no significant increase in EdU incorporation in the inner hair cells or inner phalangeal cells in Pofut1 and dnMAML1 mutant embryos compared to wild type controls. We occasionally saw EdU incorporation in border cells in dnMAML1 mutant embryos (asterisk). Data for EdU incorporation is provided in Table 1.

Supernumerary hair cells are present from the onset of hair cell differentiation in mutants that reduce Notch signaling.
We performed in situ hybridization for Atoh1 in sections of the E14 cochlea and examined the region of the cochlea where the first hair cells differentiate (arrows). The prosensory region is revealed by staining for p27kip1 (brown). In Pofut1 mutant mice, an Atoh1 doublet can be observed at the border of the prosensory domain and Kölliker’s organ. Scale bar = 50 µm.
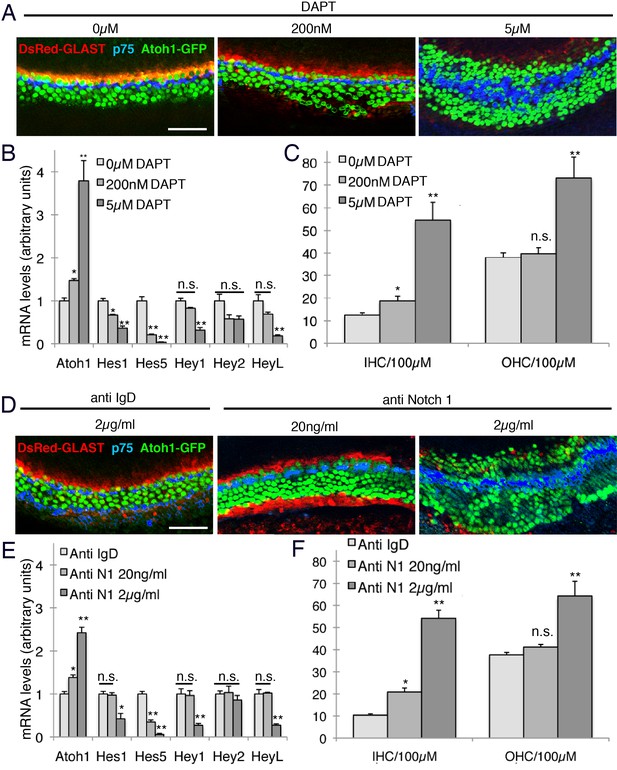
Intermediate doses of Notch inhibitors cause ectopic inner hair cells but a persistence of inner phalangeal cells.
E14 cochleas from GLAST-DsRed;Atoh1GFP/GFP mice were cultured for 24 hr in the presence of different doses of either (A) DAPT (0, 200 nM or 5 µM) or (B) Notch1 blocking antibodies (0, 20 ngml or 2 µg/ml). Inner and outer hair cells were quantified from the explants, with p75LNGFR antibody staining (blue) used to reveal pillar cells, and parallel cultures were taken for quantification of Atoh1 mRNA and the Hes/Hey genes Hes1, Hes5, Hey1, Hey2 and Heyl. For both DAPT and Notch1 antibodies, intermediate doses caused a significant increase in inner hair cell numbers but not outer hair cells, and a persistence of DsRed-expressing inner phalangeal cells (p<0.05; Student two-tailed t test).
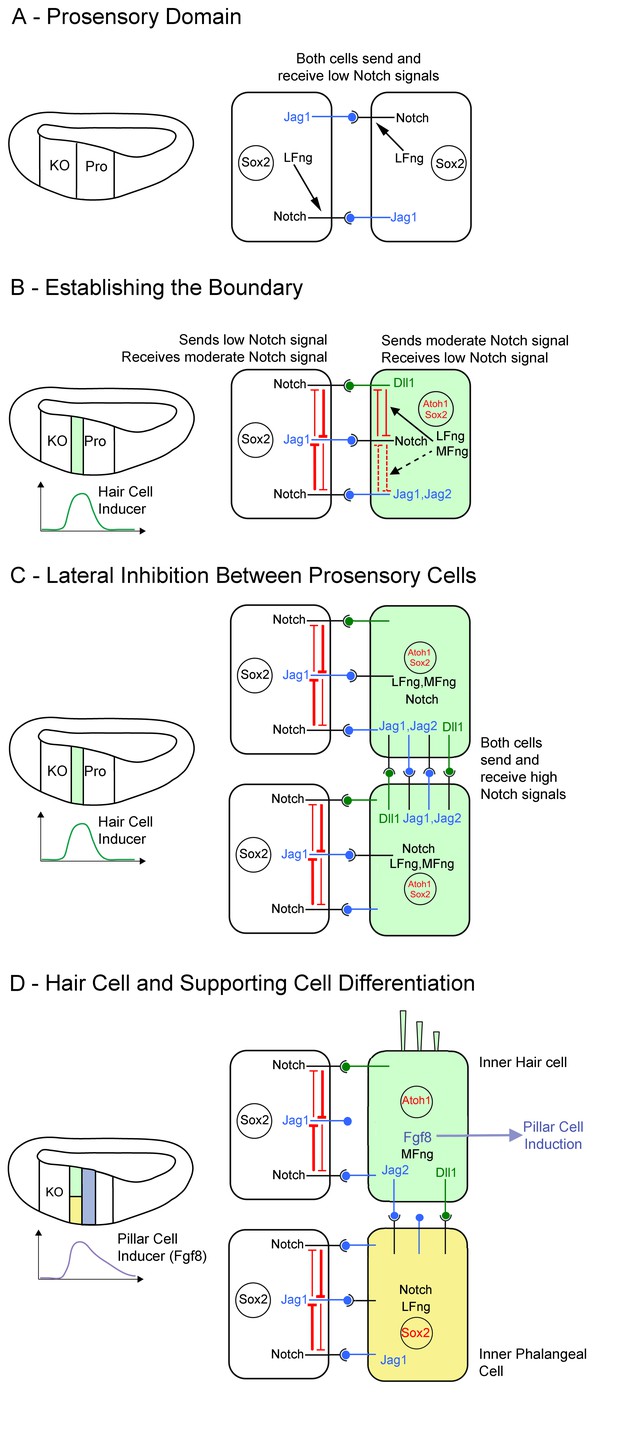
Model of Notch function during boundary formation between the prosensory domain and Kölliker’s organ.
(A): Between E11 and E13, cells in the cochlear primordium express both Lfng and Jag1 in the presumptive Kölliker’s organ (KO) and prosensory domain (Pro). Lfng-mediated attenuation of Jag1-Notch1 signaling in trans leads to very low levels of Notch signaling in these cells. (B): Starting in the base of the cochlea at E13.5, hair cell inducing signals peak at the boundary of Kölliker’s organ and the prosensory domain, leading to the up-regulation of Mfng, Jag2, Dll1, Dll3 and Atoh1 in a column of cells at the boundary (light green). We predict that the co-expression of Lfng and Mfng in these cells modulates the activity of Dll1 and Jag ligands in these cells through cis-inhibition (black arrows), The expression of Lfng and Mfng in these cells also makes them less sensitive to Jag1 signaling from neighboring cells in Kölliker’s organ (white cell). (C): As a column of hair cell progenitors is differentiating at the prosensory-Kölliker’s organ boundary (light green cells), lateral inhibition within this column of cells is carried out by Dll and Jag ligands. This typical lateral inhibition leads to (D) segregation into an inner hair cell (light green) and a supporting cell (yellow). The presence of Lfng and Mfng in this column leads to strong signaling from Dll1, and weak signaling from Jag1 and Jag2. The differentiating inner hair cells begin to express Fgf8, which induces neighboring cells to adopt a pillar cell fate (purple).
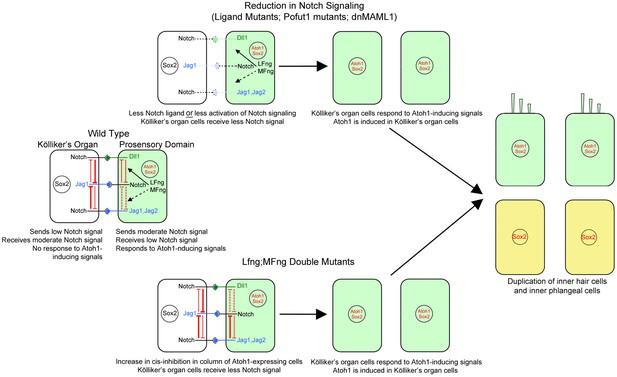
Mechanism of the Notch pathway mutants in this study.
The wild type situation is shown on the left, where prosensory cells that receive hair cell inducing signals (green) deliver a moderate Notch signal to Kölliker’s organ cells (white) and prevent them from adopting a prosensory or hair cell fate. In Notch ligand mutants (Jag1, Jag2), prosensory cells deliver less Notch signal to Kölliker’s organ. In mutants that affect the Notch receptor (Pofut1) or intracellular Notch signaling (dnMAML1), less signal is received by Kölliker’s organ. In all cases (center, top), one row of Kölliker’s organ cells now responds to Atoh1 inducing signals and duplicate the inner hair cell and inner phalangeal cell (right image). In cells lacking Lfng and Mfng, (center, bottom), increased cis-inhibition in the prosensory region is predicted to deliver less Notch signal to Kölliker’s organ. Once again, these cells respond to Atoh1 inducing signals and duplicate the inner hair cell and inner phalangeal cell (right image).
Tables
EdU labeling of cochlear progenitor cells in two Notch loss-of-function mutants.
Total EdU labeled cell types | ||||||||
|---|---|---|---|---|---|---|---|---|
Number of cochleas | Number of sections | IHC | OHC | BC | IPC | PC | DC | |
Control | 11 | 314 | 0 (0) | 3 (0.009) | 59 (0.187) | 11 (0.035) | 2 (0.006) | 9 (0.028) |
dnMAML1 Mutant | 9 | 239 | 1 (0.004) | 5 (0.02) | 66* (0.276) | 10 (0.041) | 2 (0.008) | 5 (0.020) |
Pofut1 Mutant | 4 | 103 | 0 (0) | 1 (0.019) | 24 (0.233) | 8 (0.077) | 0 (0) | 4 (0.038) |
-
We administered EdU to pregnant female mice three times a day between E14.5 and E17.5 and collected embryos for analysis at E18.5. The total numbers of dividing cells labeled by EdU for each genotype was normalized by dividing the number of labeled cells by the total number of sections counted. The total number counted for all sections is shown under each cell type and the normalized number per section is shown below in parentheses. A modified Wald test for two-sample proportions was used to determine whether the numbers of dividing cells was significantly different in either mutant group compared with the control groups. Statistical tests were applied to individual hair and supporting cell types (see text). The only group that showed significant differences to control was the number of labeled border cells in dnMAML1 mutants (*p=0.014). IHC: Inner hair cells; OHC: Outer hair cells; BC: Border cells; IPC: inner phalangeal cells; PC: Pillar cells; DC: Deiters’ cells.





