Ion Channels: From ions to insulin
The hormone insulin is released from beta cells in the pancreas when the levels of sugar in the blood become too high. High levels of sugar cause the concentration of a molecule called ATP – the energy currency of the cell – to increase inside cells. Ion channels known as KATP channels sense changes in the levels of ATP to allow potassium ions to move out of beta cells, and thus have a central role in regulating blood sugar levels (Ashcroft, 2005). When ATP levels in the cell are high, the KATP channels close, and the resulting changes in the electrical excitability of the beta cell membranes lead to an increase in the release of insulin. Likewise, when blood sugar levels decrease, cellular ATP levels also drop, causing the KATP channels to open so that less insulin is released.
These channels are of considerable medical interest because mutations that affect them can cause diabetes or result in abnormally high levels of insulin, which causes a disease known as hyperinsulinism. Drugs that close KATP channels to stimulate the release of insulin are widely used to treat type II diabetes, while drugs that open these channels are used to treat mild forms of hyperinsulinism. Now, in eLife, James Chen and Show-Ling Shyng and colleagues at the Oregon Health and Science University – including Gregory Martin as first author – report that they have used electron cryo-microscopy (cryo-EM) to determine the structure of a KATP channel in the closed state (Martin et al., 2017). In independent work, Ning Gao, Lei Chen and colleagues at Peking University have used cryo-EM to determine the structure of a closed KATP channel under different conditions (Li et al., 2017).
A pancreatic KATP channel contains four copies of a pore-forming protein called Kir6.2 and four copies of a regulatory protein called SUR1 (Figure 1A). The SUR1 subunits control the flow of potassium ions through the pore formed by the Kir6.2 subunits. ATP closes KATP channels by binding to the Kir6.2 subunits. When blood sugar levels are low, another molecule called ADP along with magnesium ions (together known as MgADP) bind to the SUR1 subunits and the channels open. Other factors also regulate KATP channels, such as the lipid PIP2, which opens the channel (Nichols, 2006).
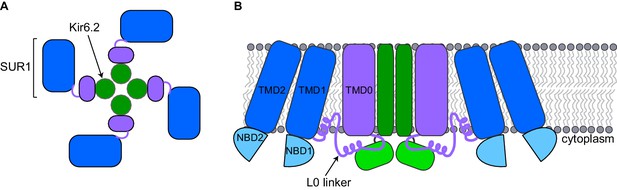
Schematic diagrams of a closed KATP channel.
Each KATP channel contains four Kir6.2 subunits (green) and four SUR1 subunits. Each SUR1 subunit contains a core (blue), an L0 linker (purple line) and a transmembrane domain called TMD0 (purple). The diagrams are based on the cryo-EM KATP channel structures by Martin et al. and Li et al. (A) Top view of the channel showing the propeller-shaped structure. (B) Side view of the channel showing two Kir6.2 subunits and two SUR1 subunits sitting in the cell membrane (gray). The core of SUR1 contains two transmembrane domains (TMD1 and TMD2) and two nucleotide-binding domains (NBD1 and NBD2), while the L0 linker contains two short α-helices. The Kir6.2 subunit is made of two transmembrane helices (dark green rectangle) and two cytoplasmic regions (light green rectangle). Martin et al. and Li et al. found that the center of the KATP channel propeller structure is formed by the Kir6.2 subunits and the TMD0 of SUR1. The cores of the SUR1 subunits form the propeller blades, but there are unexpected spaces between the cores of adjacent SUR1 subunits that had not been observed in a previously reported structure formed under different conditions (Mikhailov et al., 2005).
The structure reported by Martin et al. was of a pancreatic KATP channel prepared in the presence of ATP and glibenclamide (a drug that is known to close the channels), while Li et al. solved the structure of the pancreatic KATP channel prepared in the presence of glibenclamide alone. However, both groups report that the closed channels have similar propeller-shaped structures (Figure 1A).
SUR1 contains a core structure of two membrane-spanning domains and two nucleotide-binding domains (Dean et al., 2001), along with an additional membrane-spanning domain known as TMD0 that is connected to the SUR1 core structure by the L0 linker (Aguilar-Bryan et al., 1995; Figure 1B). The cryo-EM structures show that the SUR1 TMD0 interacts with the Kir6.2 pore. Thus, the cryo-EM structures highlight how TMD0 is important in channel assembly, and explain why this SUR1 domain is a hot spot for mutations that cause hyperinsulinism (Aittoniemi et al., 2009). An unexpected finding is that the SUR1 cores and Kir6.2 do not directly interact, even though the nucleotide-binding domains of SUR1 (which bind to MgADP) are essential for opening the pore (Nichols, 2006) and mutations affecting these domains cause disease (Aittoniemi et al., 2009).
It was known from previous work that Kir6.2 is made of two membrane-spanning helices and two cytoplasmic regions (Figure 1; Hibino et al., 2010). The cryo-EM structures show that ATP, PIP2, and glibenclamide bind to regions of the Kir6.2 and SUR1 subunits, and hence explain why both types of subunits affect the action of these molecules. ATP binds to a site between the cytoplasmic regions of adjacent Kir6.2 subunits and a region of the SUR1 L0 linker. PIP2 binds to an adjacent site that also involves the Kir6.2 cytoplasmic domains and the SUR1 L0 linker, while glibenclamide binds at a site made by a different region of the L0 linker and the core of SUR1.
The structures highlight the dynamics involved in controlling whether the channel is open or closed. Both teams found that there is a subset of closed channels in which the cytoplasmic domains of Kir6.2 are rotated in a way that may represent a transition toward the open state. Notably, Li et al. show that the inner membrane-spanning helices of two of the Kir6.2 subunits are slightly displaced in this subset of channels, which also possess a bound PIP2 molecule.
Previous studies on proteins that structurally resemble SUR1 suggest that, for the channel to open in the presence of MgADP, the SUR1 core has to "untwist" to allow the two nucleotide-binding domains to come into contact (Wilkens, 2015). Further structural studies will be needed to find out whether the twisted SUR1 core in the closed KATP channel is caused by glibenclamide binding to the channel, and to reveal how other drugs that open KATP channels work.
Particular regions in the SUR1 transmembrane domains sense conformational changes in the nucleotide-binding domains caused by MgADP binding. Because these regions also bind to the L0 linker, it is possible that conformational changes in the nucleotide-binding domains may alter the structure of the L0 linker. Structural studies of the channels in the open state would shed more light on whether the L0 linker structure changes and may reveal how mutations in Kir6.2 and SUR1 that disrupt the ability of the channel to close can cause diabetes. The exciting structures of KATP channels reported by Martin et al. and Li et al. lay the foundation for finally answering many of these questions.
References
-
SUR1: a unique ATP-binding cassette protein that functions as an ion channel regulatorPhilosophical Transactions of the Royal Society B: Biological Sciences 364:257–267.https://doi.org/10.1098/rstb.2008.0142
-
ATP-sensitive potassium channelopathies: focus on insulin secretionJournal of Clinical Investigation 115:2047–2058.https://doi.org/10.1172/JCI25495
-
The human ATP-binding cassette (ABC) transporter superfamilyGenome Research 11:1156–1166.https://doi.org/10.1101/gr.GR-1649R
Article and author information
Author details
Publication history
- Version of Record published: March 9, 2017 (version 1)
- Version of Record updated: March 9, 2017 (version 2)
Copyright
© 2017, Kanelis
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 2,316
- views
-
- 211
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Structural Biology and Molecular Biophysics
Roco proteins entered the limelight after mutations in human LRRK2 were identified as a major cause of familial Parkinson’s disease. LRRK2 is a large and complex protein combining a GTPase and protein kinase activity, and disease mutations increase the kinase activity, while presumably decreasing the GTPase activity. Although a cross-communication between both catalytic activities has been suggested, the underlying mechanisms and the regulatory role of the GTPase domain remain unknown. Several structures of LRRK2 have been reported, but structures of Roco proteins in their activated GTP-bound state are lacking. Here, we use single-particle cryo-electron microscopy to solve the structure of a bacterial Roco protein (CtRoco) in its GTP-bound state, aided by two conformation-specific nanobodies: NbRoco1 and NbRoco2. This structure presents CtRoco in an active monomeric state, featuring a very large GTP-induced conformational change using the LRR-Roc linker as a hinge. Furthermore, this structure shows how NbRoco1 and NbRoco2 collaborate to activate CtRoco in an allosteric way. Altogether, our data provide important new insights into the activation mechanism of Roco proteins, with relevance to LRRK2 regulation, and suggest new routes for the allosteric modulation of their GTPase activity.
-
- Developmental Biology
- Structural Biology and Molecular Biophysics
A crucial event in sexual reproduction is when haploid sperm and egg fuse to form a new diploid organism at fertilization. In mammals, direct interaction between egg JUNO and sperm IZUMO1 mediates gamete membrane adhesion, yet their role in fusion remains enigmatic. We used AlphaFold to predict the structure of other extracellular proteins essential for fertilization to determine if they could form a complex that may mediate fusion. We first identified TMEM81, whose gene is expressed by mouse and human spermatids, as a protein having structural homologies with both IZUMO1 and another sperm molecule essential for gamete fusion, SPACA6. Using a set of proteins known to be important for fertilization and TMEM81, we then systematically searched for predicted binary interactions using an unguided approach and identified a pentameric complex involving sperm IZUMO1, SPACA6, TMEM81 and egg JUNO, CD9. This complex is structurally consistent with both the expected topology on opposing gamete membranes and the location of predicted N-glycans not modeled by AlphaFold-Multimer, suggesting that its components could organize into a synapse-like assembly at the point of fusion. Finally, the structural modeling approach described here could be more generally useful to gain insights into transient protein complexes difficult to detect experimentally.






