Ion Channels: Poring over furrows
Proteins known as calcium-activated chloride channels have a central role in processes as diverse as regulating blood pressure in mammals and closing the Venus flytrap (Hartzell et al., 2005). In 2008, it was discovered that the archetypal forms of these channels are encoded by a gene called TMEM16A (also known as ANO1; Caputo et al., 2008; Schroeder et al., 2008; Yang et al., 2008). Since this discovery, hundreds of papers have been published about these channels, providing important insights into the relationship between their structure and function. However, relatively little was known about the structure of the channels at the atomic level. Now, in eLife, Raimund Dutzler at the University of Zurich and colleagues – including Cristina Paulino as first author – report that they have used cryo-electron microscopy (cryo-EM) to obtain a near-atomic resolution structure of a mouse TMEM16A channel (Paulino et al., 2017).
The TMEM16 proteins are also known as anoctamins because it was first thought that all the proteins in the TMEM16A family contained eight (octa) transmembrane domains and had pores that allowed chloride ions and other anions to traverse membranes. However, the use of this name has been controversial because many of the TMEM16 proteins are not, in fact, anion channels, despite the similarity in their amino acid sequences. Rather, most of them are phospholipid scramblases that passively transport phospholipid molecules between the two lipid layers of cell membranes (Suzuki et al., 2010; Bevers and Williamson, 2016).
Phospholipids are amphipathic: they contain a hydrophilic ('water-loving') head attached to a hydrophobic ('water-hating') tail. When they assemble into membranes, the tails of the phospholipids in the outer layer orient towards the tails of the phospholipids in the inner layer so that the membrane has a hydrophobic core and hydrophilic surfaces. The Dutzler laboratory revealed how TMEM16 scramblases transport lipids between layers when they solved the X-ray structure of a fungal phospholipid scramblase known as nhTMEM16 (Brunner et al., 2014). This structure showed that these scramblases are made of pairs (dimers) of TMEM16 molecules. Each 'subunit' in the dimer has an unusual hydrophilic furrow on its surface that allows the heads of the phospholipids to move from one layer to the other while their tails remain in the hydrophobic core of the membrane.
The X-ray structure of nhTMEM16 sparked many questions about the differences between the structures of the scramblases and the ion channels. Unlike the scramblases, which transport amphipathic phospholipids, TMEM16A channels support the passage of small hydrophilic anions. It was initially hypothesized that TMEM16A subunits might form dimers with their hydrophilic grooves facing one another to create a single hydrophilic pore in the center (Brunner et al., 2014). However, this hypothesis was called into question by studies that demonstrated that TMEM16A dimers have two separate pores (Jeng et al., 2016; Lim et al., 2016). We suggested that the pore is composed of both proteins and lipids (Whitlock and Hartzell, 2016), but this model is now challenged by the new structure of mouse TMEM16A.
Paulino et al. now show that the mouse TMEM16A channel, like the nhTMEM16 scramblase, forms dimers with each subunit having 10 helices that span the membrane. Instead of being an open furrow, the ion-conducting pore in the channel is enclosed by protein along most of its length. This is due to a structural rearrangement that brings the two helices that form the edges of the furrow in nhTMEM16 together in TMEM16A to enclose the pore (Figure 1). The pore is shielded from the hydrophobic core of the membrane for about two-thirds the distance across the membrane while the remainder is open to the lipids and cytoplasm.
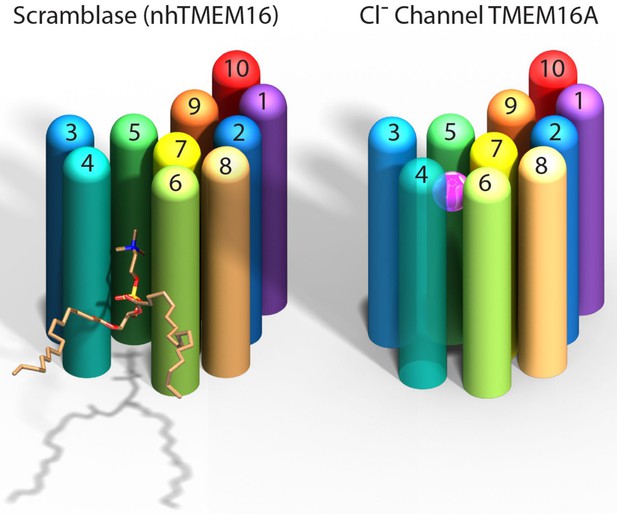
Comparison of a TMEM16 scramblase and a TMEM16 chloride channel.
The fungal nhTMEM16 protein (left), which acts as a lipid scramblase, and the mouse TMEM16A protein (right), which operates as a chloride ion channel, both contain 10 alpha helices (shown here as colored cylinders) embedded in the membrane. Only one subunit of the dimer is shown. The lower end of each protein faces into the cell, while the upper end faces outwards. While there are small differences in the relative locations of most of the helices, the biggest difference between the two proteins is the position of helix 4: in nhTMEM16 helix 4 is quite close to helix 3, which leads to the formation of a furrow by helices 4, 5 and 6; in TMEM16A helix 4 is further away from helix 3, and closer to helix 6, which leads to the formation of a narrow pore by helices 3–7. The furrow is able to transport lipids such as phosphatidylcholine (shown here), whereas the pore can transport chloride ions (pink sphere).
This model confirms the double-barreled nature of TMEM16A (Jeng et al., 2016; Lim et al., 2016): each subunit of the dimer has its own pore with the interface between the two formed largely by just one helix in each subunit. Confidence in the overall structural model is provided by experiments using mutagenesis to substitute positively charged amino acids in the pore with neutral ones. The effects of these substitutions are consistent with the idea that altering the charge of the pore will affect the ability of anions to move through the pore.
Several key questions remain. It is very likely that the TMEM16A structure described here is the conformation of the protein when it is bound to calcium ions because it was purified in the presence of high levels of calcium ions. However, it was not possible to determine the exact locations of individual amino acids within the pore, so the details of the geometry of the pore remain uncertain. Since TMEM16A readily inactivates in the presence of high levels of calcium ions (Lim et al., 2016), this structure could conceivably represent a state that does not conduct ions.
The narrowest part of the pore in the cryo-EM structure is estimated to be 3.6 angstroms wide, which is exactly the diameter of a chloride ion. This raises the question of how the pore could accommodate the passage of larger ions, such as iodide and tricyanomethanide, which are also known to be able to move through TMEM16A channels (Qu and Hartzell, 2000; Schroeder et al., 2008). In spite of this ambiguity, the findings of Paulino et al. are an essential step forward in understanding how chloride ions pass through TMEM16 channels and the evolutionary relationships between the members of this protein family.
References
-
Calcium-activated chloride channelsAnnual Review of Physiology 67:719–758.https://doi.org/10.1146/annurev.physiol.67.032003.154341
-
Independent activation of distinct pores in dimeric TMEM16A channelsThe Journal of General Physiology 148:393–404.https://doi.org/10.1085/jgp.201611651
-
Independent activation of ion conduction pores in the double-barreled calcium-activated chloride channel TMEM16AThe Journal of General Physiology 148:375–392.https://doi.org/10.1085/jgp.201611650
-
Anion permeation in Ca2+-activated Cl- channelsThe Journal of General Physiology 116:825–844.https://doi.org/10.1085/jgp.116.6.825
-
A pore Idea: the ion conduction pathway of TMEM16/ANO proteins is composed partly of lipidPflügers Archiv - European Journal of Physiology 468:455–473.https://doi.org/10.1007/s00424-015-1777-2
Article and author information
Author details
Publication history
- Version of Record published: May 31, 2017 (version 1)
Copyright
© 2017, Fisher et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,697
- views
-
- 146
- downloads
-
- 3
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Structural Biology and Molecular Biophysics
Roco proteins entered the limelight after mutations in human LRRK2 were identified as a major cause of familial Parkinson’s disease. LRRK2 is a large and complex protein combining a GTPase and protein kinase activity, and disease mutations increase the kinase activity, while presumably decreasing the GTPase activity. Although a cross-communication between both catalytic activities has been suggested, the underlying mechanisms and the regulatory role of the GTPase domain remain unknown. Several structures of LRRK2 have been reported, but structures of Roco proteins in their activated GTP-bound state are lacking. Here, we use single-particle cryo-electron microscopy to solve the structure of a bacterial Roco protein (CtRoco) in its GTP-bound state, aided by two conformation-specific nanobodies: NbRoco1 and NbRoco2. This structure presents CtRoco in an active monomeric state, featuring a very large GTP-induced conformational change using the LRR-Roc linker as a hinge. Furthermore, this structure shows how NbRoco1 and NbRoco2 collaborate to activate CtRoco in an allosteric way. Altogether, our data provide important new insights into the activation mechanism of Roco proteins, with relevance to LRRK2 regulation, and suggest new routes for the allosteric modulation of their GTPase activity.
-
- Developmental Biology
- Structural Biology and Molecular Biophysics
A crucial event in sexual reproduction is when haploid sperm and egg fuse to form a new diploid organism at fertilization. In mammals, direct interaction between egg JUNO and sperm IZUMO1 mediates gamete membrane adhesion, yet their role in fusion remains enigmatic. We used AlphaFold to predict the structure of other extracellular proteins essential for fertilization to determine if they could form a complex that may mediate fusion. We first identified TMEM81, whose gene is expressed by mouse and human spermatids, as a protein having structural homologies with both IZUMO1 and another sperm molecule essential for gamete fusion, SPACA6. Using a set of proteins known to be important for fertilization and TMEM81, we then systematically searched for predicted binary interactions using an unguided approach and identified a pentameric complex involving sperm IZUMO1, SPACA6, TMEM81 and egg JUNO, CD9. This complex is structurally consistent with both the expected topology on opposing gamete membranes and the location of predicted N-glycans not modeled by AlphaFold-Multimer, suggesting that its components could organize into a synapse-like assembly at the point of fusion. Finally, the structural modeling approach described here could be more generally useful to gain insights into transient protein complexes difficult to detect experimentally.






