Jak2-mediated phosphorylation of Atoh1 is critical for medulloblastoma growth
Figures
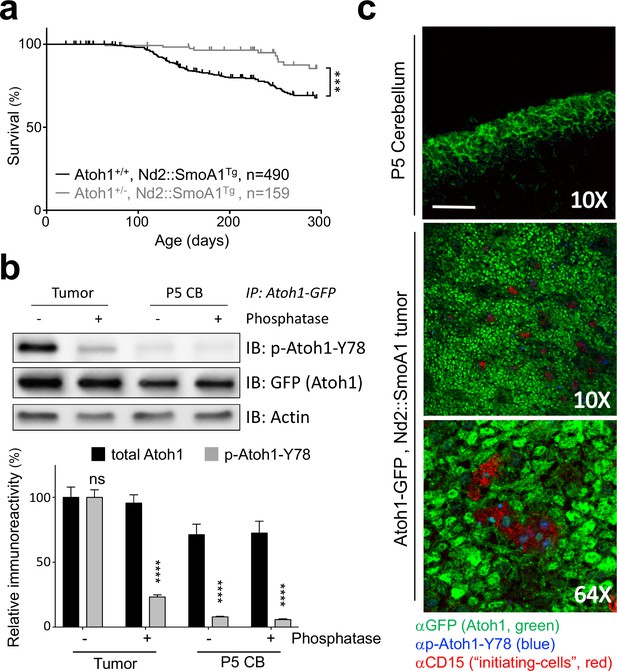
Tyrosine 78 of Atoh1 protein is phosphorylated in MB-initiating cells in vivo.
(a) Kaplan-Meier survival curve shows that Atoh1 heterozygosity markedly reduces MB tumor incidence in mice (Log Rank (Mantel-Cox)). (b) Atoh1 tyrosine 78 is strongly phosphorylated in malignant but not healthy cerebellar tissue (n = 3, representative blot shown, Two-way ANOVA with Sidak's multiple comparisons test). (c) Y78 (blue) is phosphorylated in the tumor-initiating cell (CD15+, red) islets. Values are mean ± s.e.m.; ***p<0.001, ****p<0.0001, scale = 200 µm.
-
Figure 1—source data 1
This excel file contains all relevant statistical analyses for the manuscript.
- https://doi.org/10.7554/eLife.31181.005
-
Figure 1—source data 2
Atoh1 tyrosine 78 coverage and Atoh1 interacting proteins.
- https://doi.org/10.7554/eLife.31181.006
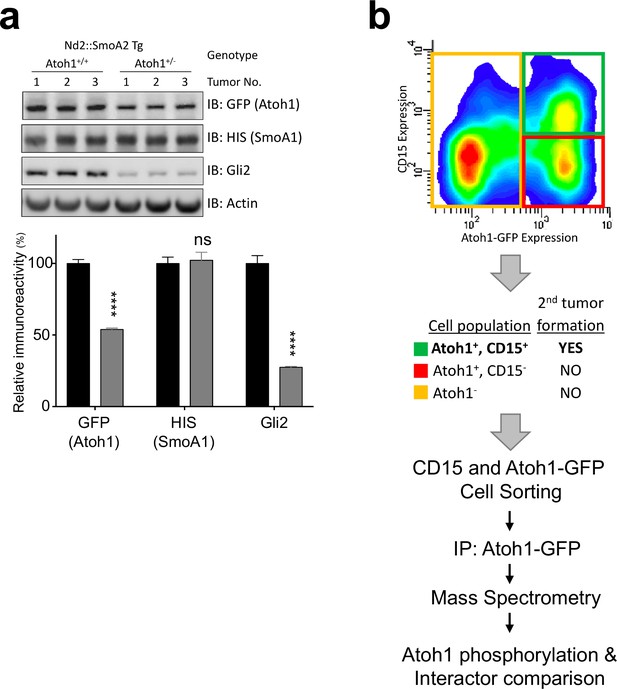
Atoh1 is required for tumor-initiating cells.
(a) Western blot analysis of Atoh1-GFP homozygous or heterozygous Nd2::SmoA1 transgenic mice with quantification to the right. n = 6 (b) FACS blot of Atoh1-GFP homozygous, Nd2::SmoA1 tumor. After sorting the three indicated cell population, only the Atoh1/CD15 double positive cell population (green) was able to generate flank tumors. Thus, Atoh1+/CD15+ and CD15- (red) populations were used in the IP-MS experiments outlined below. ****p<0.0001.
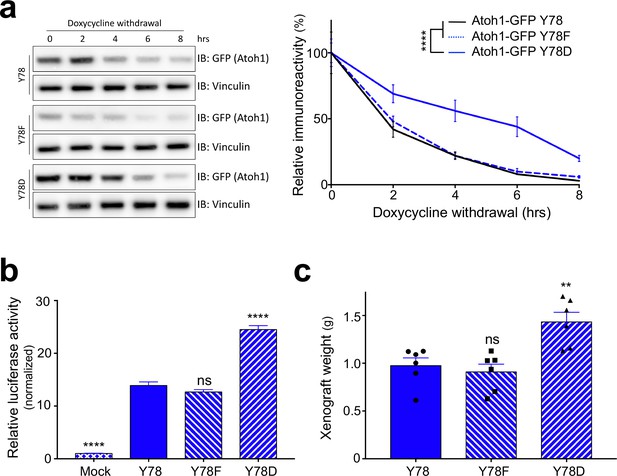
Y78 phosphorylation regulates Atoh1 levels.
(a) Y78 phosphorylation increases Atoh1 stability in doxycycline inducible, stable DAOY cell lines (n = 3, representative blot shown, non-linear regression). (b) Y78 phosphorylation increases transcriptional activity of Atoh1-specific reporter in DAOY cells (n = 46 in duplicates, One-way ANOVA compared to Y78, Dunnett's multiple comparisons test). (c) Y78 phosphorylation significantly increases tumor growth in grafted, Atoh1-transduced DAOY cells three months after implantation (n = 6, One-way ANOVA compared to Y78). Y78 = Atoh1-GFP WT, Y78F = Atoh1-GFP Y78F, Y78D = Atoh1-GFP Y78D; values are mean ± s.e.m.; **p<0.01, ****p<0.0001, ns = not significant.
-
Figure 2—source data 1
Atoh1 half-life analysis based on stably expressing cell lines.
- https://doi.org/10.7554/eLife.31181.010
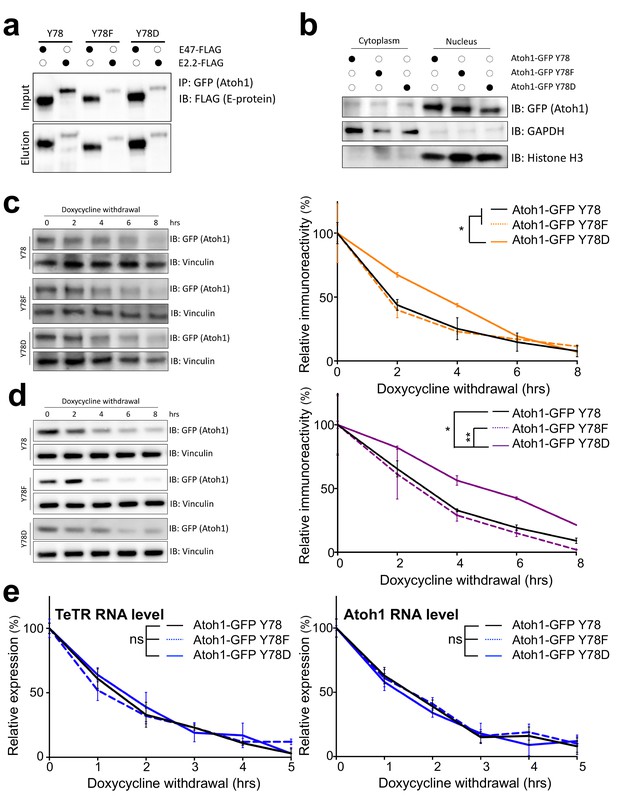
Tyrosine 78 molecular consequences.
(a) Co-IP of Atoh1 mutants with two obligate binding partners, E47 or E2.2 in DAOY cells transfected for 48 hr. n = 3. (b) Nuclear (marked by Histone H3) and cytoplasmic (marked by GAPDH) fractionation of DAOY cells transfected with one of the three Atoh1 mutants for 48 hr. n = 3. (c–d) Stability analysis of three Atoh1 transgenic cell lines based on UW228 (c) or ONS-76 (d) parental cell lines. n = 3. (e) RNA stability of Atoh1 transgenes, as well as the TeTR transgenes in DAOY cell lines. n = 3. Y78 = Atoh1-GFP WT, Y78F = Atoh1-GFP Y78F, Y78D = Atoh1-GFP Y78D; *p<0.05, **p<0.01.
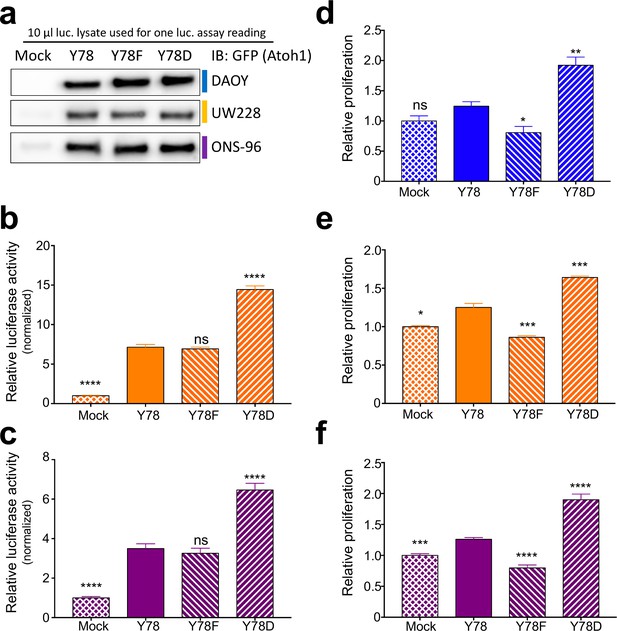
Tyrosine 78 biological consequences.
(a) Western blot analysis of dual luciferase reporter lysates. The same amount of lysate was analysed, which corresponds to one luciferase reading. (b–c) Dual luciferase reporter assay using UW228 (b, orange) or ONS-76 (c, purple) cell lines transfected with corresponding Atoh1 mutants and Atoh1-specific firefly reporter. n = 46 in duplicates. (d–f) in vitro proliferation assay using medullospheres of DAOY (d, blue), UW228 (e, orange), or ONS-76 (f, purple) stably infected cell lines. n = 3 in triplicates. Y78 = Atoh1 GFP WT, Y78F = Atoh1 GFP Y78F, Y78D = Atoh1 GFP Y78D; *p<0.05, **p<0.01, ***p<0.001, ****p<0.0001, ns denotes p>0.05.
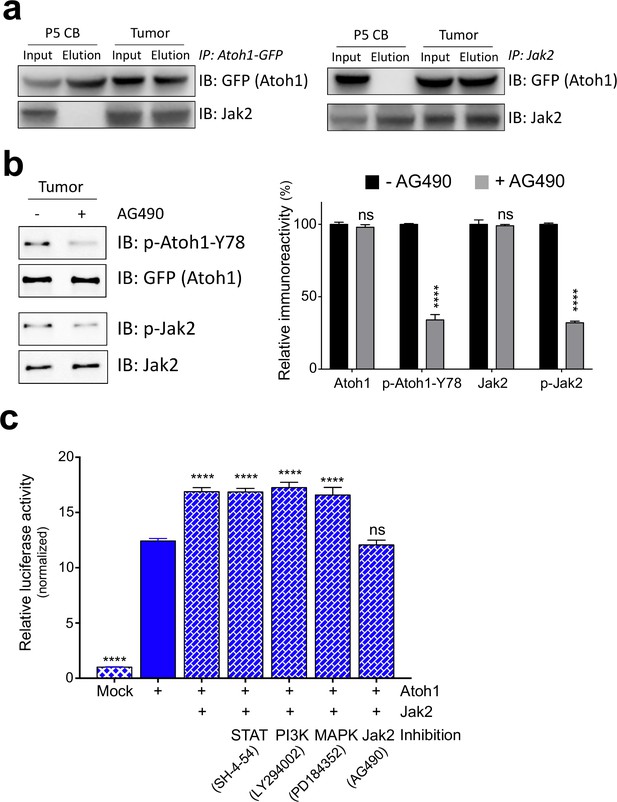
Jak2 phosphorylates Atoh1 Y78.
(a) IP of Jak2 or Atoh1 in tumor and P5 cerebellar tissue shows that Atoh1 and Jak2 interact in tumor tissue. (b) Upon inhibition of Jak2, Y78 phosphorylation levels fall dramatically in ex vivo culture (n = 3, Two-way ANOVA with Sidak's multiple comparisons test). (c) Transcriptional activity of Atoh1 is increased upon Jak2 introduction, independent of canonical Jak2 pathways (n = 46 in duplicates, One-way ANOVA with Dunnett's multiple comparisons test compared to Atoh1-GFP). Values are mean ± s.e.m.; ****p<0.0001, ns = not significant.
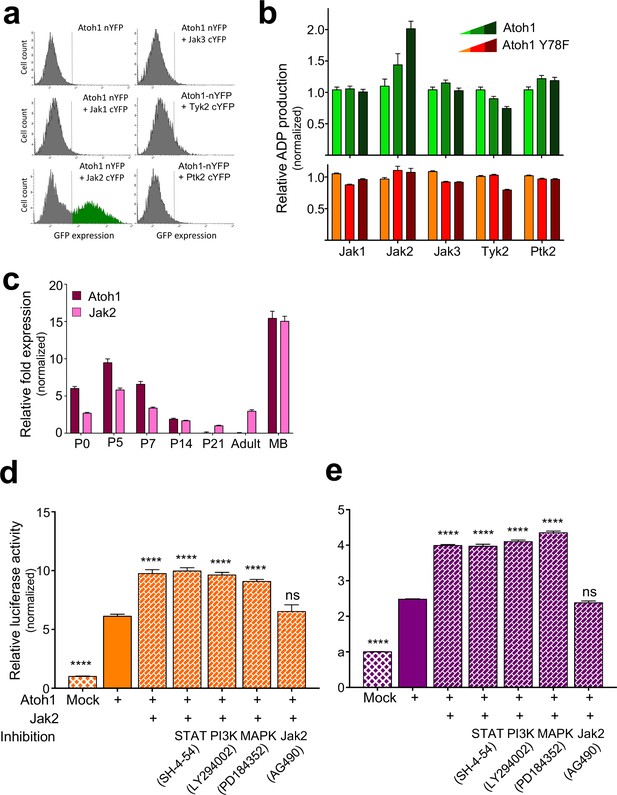
Jak2 phosphorylates tyrosine 78.
(a) FACS analysis of GFP signal intensity generated by BiFC complementation assay using DAOY cells transfected with the indicated plasmids. n = 3. (b) In vitro, non-radioactive kinase assay of in vitro transcribed kinase/substrates. n = 3. (c) qRT-PCR analysis of Atoh1 and Jak2 expression in P5, adult cerebellum, and Nd2::SmoA1 tumors, relative to P5 expression, normalized to the housekeeping gene GAPDH. n = 3. (d–e) Dual luciferase reporter assay using UW228 (d, orange) or ONS-76 (e, purple) cell lines transfected with the indicated plasmids and treated with the indicated inhibitors of the canonical Jak2 cascades. n = 46. ****p<0.0001, ns denotes p>0.05.
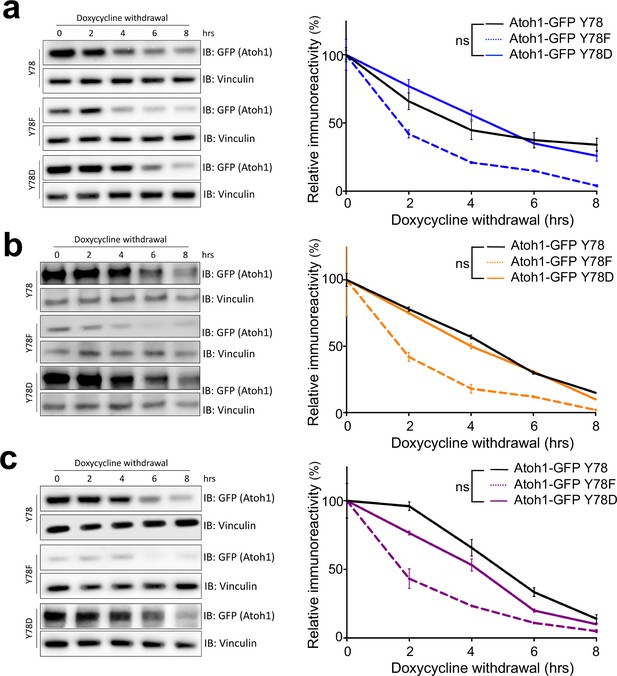
Jak2 increases Atoh1 stability.
Stability assay of stable cell lines infected with Jak2. in DAOY (a, blue), UW228 (b, orange), or ONS-76 (c, purple) parental cell lines. n = 3. Y78 = Atoh1-GFP WT, Y78F = Atoh1-GFP Y78F, Y78D = Atoh1-GFP Y78D; *p<0.05, ns denotes p>0.05.
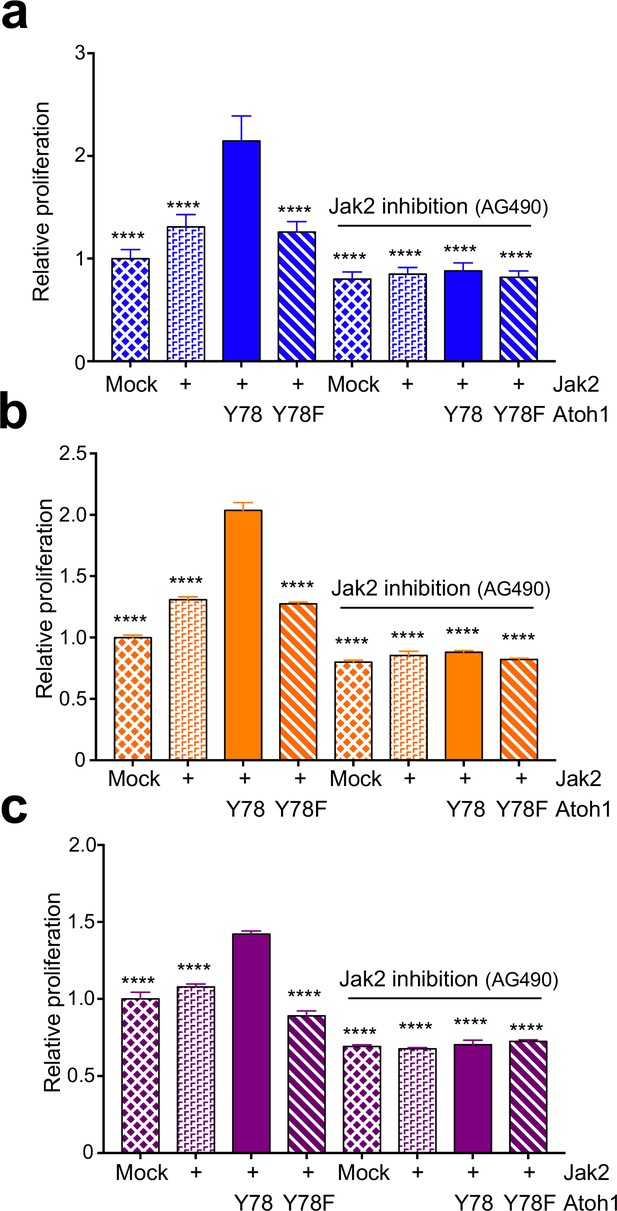
Jak2 increases proliferation via tyrosine 78.
In vitro proliferation assay using medullospheres of DAOY (a, blue), UW228 (b, orange), or ONS-76 (c, purple) cell lines stably expressing indicated Atoh1 constructs and infected with Jak2 viral particles as indicated. n = 3 in triplicates. Y78 = Atoh1-GFP WT, Y78F = Atoh1-GFP Y78F; ****p<0.0001.
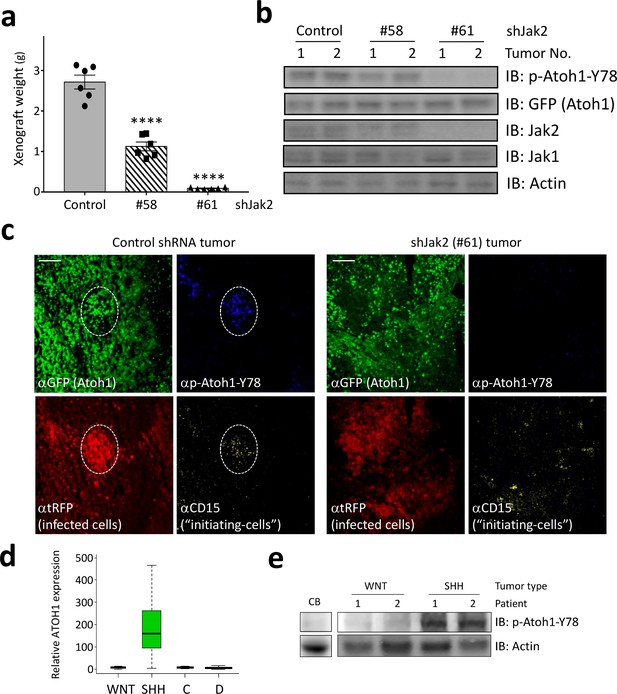
Jak2 inhibition reduces tumor growth in vivo.
(a–b) In xerograph experiments, inhibition of Jak2 via shRNA inhibits tumor growth (n = 6, One-way ANOVA with Dunnett's multiple comparisons test) (a), which is paralleled by loss of Y78 phosphorylation (b). (c) shJak2-infected tumor cells display neither defined initiating islets nor Y78 phosphorylation. (d) Box plot of human ATOH1 expression showing that Atoh1 is expressed only in the SHH subtype and no other subtypes. Data were taken from Kool et al., 2012. (e) Human MB tissue displays Y78 phosphorylation in SHH but not WNT-type tumors or post-mortem adult cerebellar tissue. Values are mean ± s.e.m.; ****p<0.0001, scale = 200 µm.
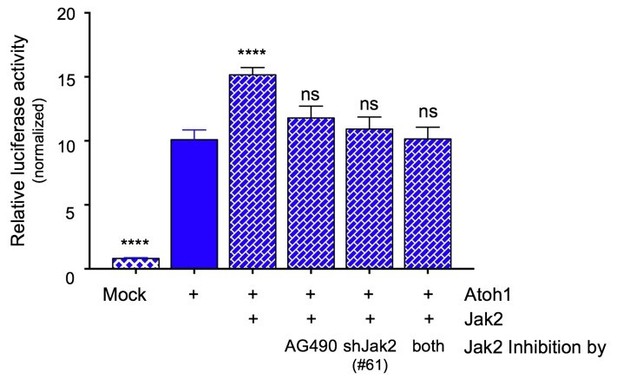
Jak2 inhibition either by AG490 or knock down using shRNA (#61) similarly prevents the increase in transcriptional activity of an Atoh1-specific reporter in DAOY cells.
Combining the inhibitor with genetic knock down does not result in any further inhibition of Atoh1 activity, consistent with Jak2 being the major component responsible for the modulation of Atoh1 (n=36 in duplicates, One-way ANOVA compared to Y78, Dunnett's multiple comparisons test. ****p<0.0001, ns=not significant).
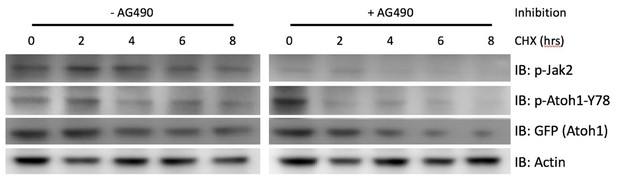
Inhibition of Jak2 by AG490 results in the destabilization of Atoh1 in primary Shh-type medulloblastoma cells.
Isolated primary mouse Shh-type medulloblastoma cell were plated in a 24-well format and treated with Cyclohexamide (CHX) three hours after plating for the indicated durations. Another set of cells were additionally treated with AG490 to inhibit Jak2 activation. AG490 treatment resulted in a dramatic reduction in Jak2 and tyrosine 78 phosphorylation within 2 hrs, as well as a reduction in Atoh1 stability. n=3 in duplicates.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| DAOY cell line (human) | inducible Atoh1-GFP WT | this paper | N/A | transgenic cell line which expresses mouse Atoh1 mutants or wildtype, tagged with GFP upon dox treatment. |
| DAOY cell line (human) | inducible Atoh1-GFP Y78F | this paper | N/A | transgenic cell line which expresses mouse Atoh1 mutants or wildtype, tagged with GFP upon dox treatment. |
| DAOY cell line (human) | inducible Atoh1-GFP Y78D | this paper | N//A | transgenic cell line which expresses mouse Atoh1 mutants or wildtype, tagged with GFP upon dox treatment. |
| UW228 cell line (human) | inducible Atoh1-GFP WT | this paper | N/A | transgenic cell line which expresses mouse Atoh1 mutants or wildtype, tagged with GFP upon dox treatment. |
| UW228 cell line (human) | inducible Atoh1-GFP Y78F | this paper | N/A | transgenic cell line which expresses mouse Atoh1 mutants or wildtype, tagged with GFP upon dox treatment. |
| UW228 cell line (human) | inducible Atoh1-GFP Y78D | this paper | N/A | transgenic cell line which expresses mouse Atoh1 mutants or wildtype, tagged with GFP upon dox treatment. |
| ONS-76 cell line (human) | inducible Atoh1-GFP WT | this paper | N/A | transgenic cell line which expresses mouse Atoh1 mutants or wildtype, tagged with GFP upon dox treatment. |
| ONS-76 cell line (human) | inducible Atoh1-GFP Y78F | this paper | N/A | transgenic cell line which expresses mouse Atoh1 mutants or wildtype, tagged with GFP upon dox treatment. |
| ONS-76 cell line (human) | inducible Atoh1-GFP Y78D | this paper | N/A | transgenic cell line which expresses mouse Atoh1 mutants or wildtype, tagged with GFP upon dox treatment. |
| recombinant DNA reagent | Atoh1-GFP_pcDNA3mod | this paper | N/A | Construct for overexpression in cell culture |
| recombinant DNA reagent | Atoh1-GFP-Y78F_pcDNA3mod | this paper | N/A | Construct for overexpression in cell culture |
| recombinant DNA reagent | Atoh1-GFP-Y78D_pcDNA3mod | this paper | N/A | Construct for overexpression in cell culture |
| recombinant DNA reagent | E47-FLAG_pcDNA3mod | this paper | N/A | Construct for overexpression in cell culture |
| recombinant DNA reagent | E2.2-FLAG_pcDNA3mod | this paper | N/A | Construct for overexpression in cell culture |
| recombinant DNA reagent | 3x AtEAM_pGL4.23 | this paper | N/A | Luciferase reporter construct |
| recombinant DNA reagent | Atoh1-GFP_pINDUCER | this paper | N/A | Construct for virus production |
| recombinant DNA reagent | Atoh1-GFP_Y78F_pINDUCER | this paper | N/A | Construct for virus production |
| recombinant DNA reagent | Atoh1-GFP-Y78D_pINDUCER | this paper | N/A | Construct for virus production |
| recombinant DNA reagent | JaK2-FLAG_pcDNA3mod | this paper | N/A | Construct for overexpression in cell culture |
| recombinant DNA reagent | Atoh1-nYFP_pBiFC | this paper | N/A | Construct for overexpression in cell culture for interaction analysis |
| recombinant DNA reagent | Jak1-cYFP_pBiFC | this paper | N/A | Construct for overexpression in cell culture for interaction analysis |
| recombinant DNA reagent | Jak2-cYFP_pBiFC | this paper | N/A | Construct for overexpression in cell culture for interaction analysis |
| recombinant DNA reagent | Jak3-cYFP_pBiFC | this paper | N/A | Construct for overexpression in cell culture for interaction analysis |
| recombinant DNA reagent | Tyk2-cYFP_pBiFC | this paper | N/A | Construct for overexpression in cell culture for interaction analysis |
| recombinant DNA reagent | Ptk2-cYFP_pBiFC | this paper | N/A | Construct for overexpression in cell culture for interaction analysis |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.31181.016





