Synergy in anti-malarial pre-erythrocytic and transmission-blocking antibodies is achieved by reducing parasite density
Figures
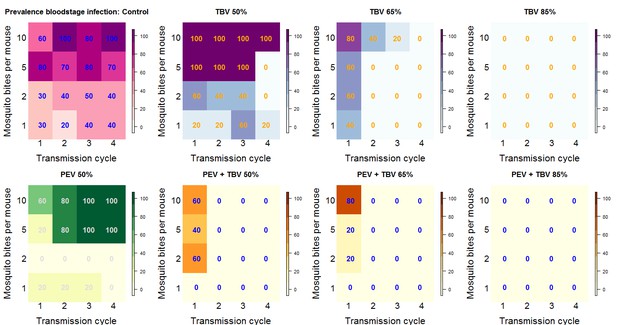
Summary outcome data.
The number in each box (and the color) shows the percentage of mice infected for each treatment arm of the experiment by transmission cycle and biting rate (the number of potentially infectious mosquito bites received per mouse). In transmission cycle 0, all mice are infected (not shown), where a TBV antibody is administered (top row: purple and blue) infections in mice are progressively reduced. The antibody works best at the higher dose (TBV 85%) and lower biting rates. The PEV antibody (lower row: yellow and green) is effective at lower biting rates (where each mouse received one or two potentially infectious mosquito bites per transmission cycle) but is not able to reduce infection at higher biting rates. In combination, at any biting rate, the combined antibodies (lower row: orange) always cleared infections by transmission cycle 2.
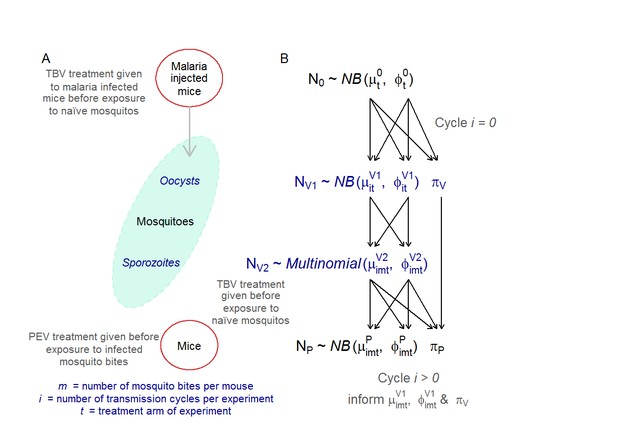
Adapted from Malaria Journal (Sherrard-Smith et al., 2017) A graphical outline of the multi-generational transmission experiment (A) and its mathematical representation (B).
(A) The mosquito-mouse model system. Five female (TO) mice (6–8 weeks old, Harlan, UK) were treated with phenylhydrazine, and, 3 days later, were infected with 106 P. berghei PbPfs25DR3 (Goodman et al., 2011). Three days later, groups of infected mice were treated with the TBV antibody. After 1 hr, the mice were anaesthetised and 500 An. stephensi mosquitoes (line SD 500, starved for 24 hr) fed randomly on the five infected mice within each group. Mosquitoes were maintained as described in (Blagborough et al., 2013). After 10 days, a sub-sample of 50 mosquitoes were microscopically examined to measure oocyst intensity and prevalence. After 21 days post-feeding, sporozoites are present in the salivary glands and are maximally infectious to the vertebrate host (Blagborough et al., 2013). At this point, pre-defined numbers of mosquitoes (to simulate mosquito biting rates of 1, 2, 5 and 10 mosquito bites per mouse) were then randomly selected from the remaining mosquitoes and fed, for 20 min, on anesthetized mice from a naive cohort. Each group of mice (five mice per group) either received the PEV antibody or no -intervention (negative control). Engorged mosquitoes were microscopically examined immediately after feeding to determine the number of sporozoites in the salivary glands. After 10 days, blood smears from each mouse were microscopically examined to determine the percentage parasitemia. These five mice were then given either the TBV antibody at the desired dose to achieve a 50%, 65% or 85% reduction in oocyst prevalence as required, or no intervention/control (in accordance with the respective treatment arm). A new cohort of 500 naive mosquitoes was then allowed to blood feed on the mice. This mouse-to-mouse transmission cycle was repeated to a maximum of four cycles after the seeding mouse population or until no parasites had been detected in the system for two successive transmission cycles. (B) The statistical model mirrored the experimental set up. The initial parasite density generated by injecting mice N0, the oocyst intensity O’ and the parasite density in mice transmitted by mosquito bites N, are modelled assuming zero-inflated negative binomial distributions. The sporozoite count S data for the biting mosquitoes were censored which means that the data are modelled as a multinomial distribution. These distributions are defined by the mean (µ, o, s for parasite density in mice, oocyst counts and sporozoite counts in mosquitoes) and dispersion (φ, τ, σ for parasite density in mice, oocyst counts and sporozoite counts in mosquitoes) and zero-inflation (πP, πV) parameters. Parameters from the previous life stage are used to inform the next (the respective parameter informing the subsequent life-stage is indicated by the arrows). The biting effect m is modelled when sporozoites in mosquitoes propagate parasite infections in mice for each transmission cycle i and treatment arm t. All care and handling of animals strictly followed the Guidelines for Animal Care and Use prepared by Imperial College London and was performed under the UK Home Office Licence 70/7185. Supplementary information Table 1—source data 1 (separate excel file). Data file of the raw data for parasite densities in mice and mosquitoes.
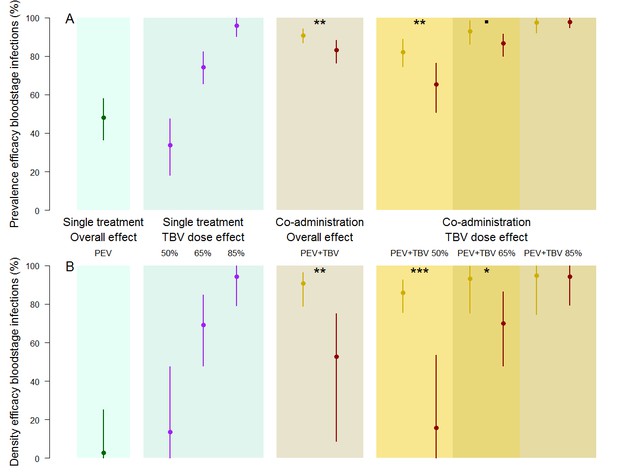
The efficacy of vaccine antibody combinations against parasite prevalence (the proportion of infected hosts) (A) and density (the mean parasite density per host, measured as the number of infected red blood cells out of a total subsample of 1200 erythrocytes) (B) in mice.
The observed efficacy of PEV monoclonal antibody mAb-3D11 at a dose previously shown to reduce transmission to mice by ~50% when exposed to five infectious mosquito bites. The efficacy of the TBV mAb-4B7 at doses previously shown to reduce transmission to mosquitoes by 50%, 65% and 85% (blue section, purple lines). The efficacy of antibodies administered together (gold sections) that were observed (gold) or expected (red) were efficacies for each antibody acting independently (mean (point) and 95% credible intervals (lines) shown). Asterisks indicate increasing levels of support of synergy (p<0.1•, p<0.05*, p<0.01**, p<0.001***).
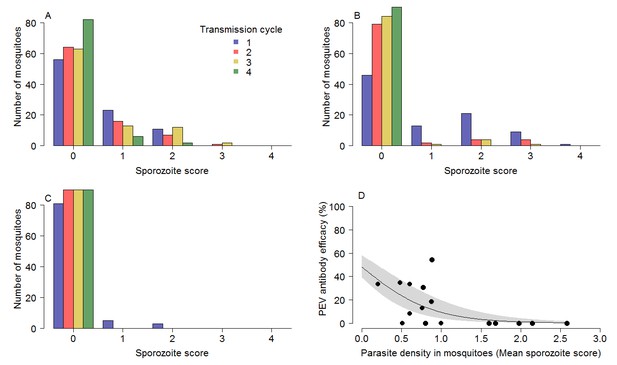
The impact of TBV monoclonal antibody mAb-4b7 on PEV mAb-3D11 efficacy.
The TBV antibody alone reduces the tail of the sporozoite distribution in mosquitoes across transmission cycles for the (A) 50% dose, (B) 65% dose and (C) 85% dose. (A–C) demonstrate that the sporozoite scores (measured on a log scale; 0 indicates no sporozoites; 1 = 1–10 sporozoites; 2 = 11–100 sporozoites; 3 = 101–1000 sporozoite and; 4 =>1000 sporozoites per mosquito) tend toward zero for successive transmission rounds. (D) The efficacy of the PEV antibodies to prevent mosquito-to-mouse-to-mosquito transmission (a percentage reduction in sporozoite infections in mosquitoes) is greatest at lower mosquito parasite densities (as assessed by sporozoite score following blood-feeding).
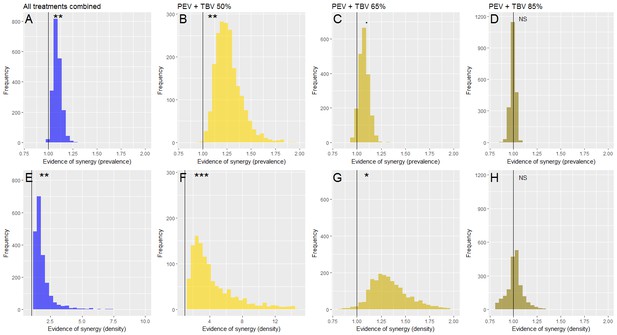
Evidence of a synergistic effect of parasite prevalence (A–D) or parasite density (E–H) of combining TBV monoclonal antibody (mAb-4B7) with PEV mAb-3D11.
The frequency histograms show the probability that antibodies administered together have a higher efficacy than if they acted independently. In all panels, the vertical black line highlights the value 1, above this line indicates synergy, below it denotes an antagonistic interaction and falling at one indicates that the antibodies are acting independently (evidence for synergy p<0.1., p<0.05*, p<0.01**, p<0.001***). Taking all data together (A and E), there is a synergistic effect of combining vaccine antibodies as the majority of iterations fall above 1. This effect is stronger at lower doses of mAb-4B7 (B, C, F and G, note the different x-axes values indicating stronger support for lower TBV antibody doses), and stronger against parasite density (E–H) than parasite prevalence.
Tables
Summary of the density model estimation of efficacy against prevalence and parasite density for the transmission blocking (TBV) and pre-erythrocytic (PEV) antibodies that were administered either alone or together to reduce malaria parasites in mice.
The interaction between the two antibodies are measured using the ratio of observed estimates for combination treatments compared to the expected efficacy were vaccine antibodies acting independently using the simulated posterior draws from the density model (Sherrard-Smith et al., 2017). A value of less than one indicates an antagonistic interaction, = 1 suggests antibodies are acting independently, and greater than one shows synergy.
| Intervention arm | Efficacy | Synergy | ||||
|---|---|---|---|---|---|---|
| Reduction in prevalence (95% credible intervals) | Reduction in density (95% credible intervals) | Prevalence | p-value | Density | p-value | |
| Individual vaccine efficacies | ||||||
| All TBV combined | 68.0 (61.1–74.1) | 51.5 (6.8–72.9) | ||||
| TBV: MAb-4B7 (50%) | 33.9 (18.2–47.4) | 13.6 (0–47.5) | ||||
| TBV: MAb-4B7 (65%) | 74.3 (65.7–82.4) | 69.3 (47.8–84.8) | ||||
| TBV: MAb-4B7 (85%) | 95.8 (90.2–100) | 94.2 (79.1–100) | ||||
| PEV: Mab-3D11 (50%) | 48.0 (36.6–58.0) | 2.8 (0–25.2) | ||||
| Combined vaccine efficacies | ||||||
| PEV and All TBV combined | 90.8 (86.9–94.2) | 90.9 (79.0–96.4) | 1.09 (1.02–1.18) | p<0.0035 | 2.08 (1.20–5.02) | p<0.0025 |
| PEV (50%) and MAb-4B7 (50%) | 82.2 (74.6–88.9) | 85.8 (75.6–92.8) | 1.27 (1.07–1.60) | p<0.0015 | 19.04 (1.56–75.16) | p<0.0001 |
| PEV (50%) and MAb-4B7 (65%) | 92.8 (86.1 - 98.6) | 93.2 (75.4 - 99.7) | 1.07 (0.98 - 1.17) | p<0.0675 | 1.36 (1.02 - 1.97) | p<0.02 |
| PEV (50%) and MAb-4B7 (85%) | 96.9 (91.2 – 100) | 94.8 (74.4 - 100) | 0.99 (0.93 - 1.04) | p<0.5435 | 1.01 (0.78 - 1.22) | p<0.3755 |
-
Table 1—source data 1
The raw data used for analysis.
- https://doi.org/10.7554/eLife.35213.006
| Resource type (species) or resource | Designation | Reference | Identifiers |
|---|---|---|---|
| Antibody | mAb-4b7 | (Stura et al., 1994) | RRID:AB_2728658 |
| Antibody | mAb-3D11 | (Mishra et al., 2012) | RRID:AB_2728657 |
Additional files
-
Transparent reporting form
- https://doi.org/10.7554/eLife.35213.010





