Bacterial Evolution: The road to resistance
Think about the last time you or a loved one had a bacterial infection and needed antibiotics. What if the antibiotics hadn’t worked? Unfortunately, this is becoming increasingly common around the world, causing at least 700,000 deaths per year (O’Neill, 2016). Resistance to antimicrobial treatments, also known as AMR, evolves rapidly, often over the course of a single infection. It occurs either by bacteria exchanging with one another the genes that confer resistance, or by an individual bacterium becoming resistant through mutations in its own genes. Resistant bacteria are more likely to survive antimicrobial treatments and go on to spread the infection to other people.
During infections, bacteria tend to grow on surfaces in communities called biofilms (Koo et al., 2017). Biofilms promote communication and cooperation, and physically shield bacteria from antimicrobials and the host immune system. These properties make biofilm infections hard to treat, even in the absence of AMR. It has been suggested that bacteria growing in biofilms may evolve AMR more rapidly, making treatment outcomes harder to predict. However, few studies have explored how different modes of growth influence the ability of bacteria to evolve (Steenackers et al., 2016).
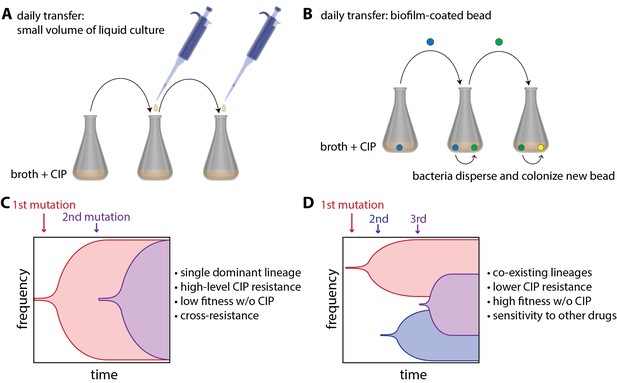
Now, in eLife, Vaughn Cooper and colleagues at the University of Pittsburg — including Alfonso Santos-Lopez and Christopher Marshall as joint first authors — report how 'bacterial lifestyle' affects the evolution of resistance to an antibiotic called ciprofloxacin (CIP) in the pathogen Acinetobacter baumannii (Santos-Lopez et al., 2019). To do this, they compared bacteria that had been floating in liquid culture as they grew to bacteria that had been growing as biofilms. The bacteria growing in liquid culture were experimentally evolved by transferring small amounts of culture to a new container with fresh growth medium every day (Figure 1A). Biofilms were evolved by growing A. baumannii on plastic beads submerged in liquid culture, and transferring these biofilm-coated beads to a new container with fresh medium and a clean bead each day (Figure 1B). At the start of the experiment, cells were exposed to a very low dose of CIP, and the CIP dose was then doubled every 72 hours as resistance evolved over a 12 day period.
Experimental evolution of resistance to ciprofloxacin (CIP) in liquid-grown and biofilm-grown bacteria.
To understand how bacterial lifestyle influences the evolution antimicrobial resistance, strains of the bacterium A.baumannii were experimentally evolved either in liquid culture (A) or as biolfims on plastic beads (B). Santos-Lopez et al. found that liquid-grown and biofilm-grown strains of A.baumannii evolved resistance to the antimicrobial CIP in different ways: liquid-grown bacteria evolved a single resistant lineage that dominated the population (C), whereas biofilm-grown bacteria evolved multiple resistant lineages that co-exist within the population (D). Other important characteristics of the two evolved strains (such as resistance to CIP) are discussed in the main text.
Samples of bacteria were collected from each culture at various times during the experiment and also at the end of the experiment. Whole genome sequencing of these samples revealed that liquid-grown and biofilm-grown A. baumannii take different, but repeatable, paths during experimental evolution. For the bacteria grown in liquid culture, Santos-Lopez et al. found that the most resistant CIP lineage outcompetes the other bacterial lineages, allowing its offspring to rapidly take over the population (Figure 1C). However, for the bacteria grown in biofilms, the researchers found that there was less competition between lineages – presumably because they are not as free to move as the liquid-grown bacteria – and that multiple co-existing lineages were able to emerge (Figure 1D).
Further experiments showed that liquid-grown and biofilm-grown bacteria had evolved unique strategies to resist CIP, resulting in different degrees of CIP resistance. Liquid-grown bacteria evolved low-level CIP resistance via mutations that increased the number of pump proteins that transport CIP out of cells. These resistant cells took over rapidly and then gained additional mutations that prevented CIP from binding to its target proteins. Bacteria with both types of mutations could resist doses of CIP that were far higher than those used during the evolution experiment. Biofilm-grown bacteria also evolved low-level CIP resistance through increased pump protein production but used different pumps than their liquid-evolved counterparts. However, biofilm-grown bacteria failed to evolve high-level resistance through additional mutations. The selective pressures imposed by the different lifestyles seem to interact with the pressure of CIP exposure and limit the evolutionary pathways, and thus outcomes, available to each lifestyle.
Santos-Lopez et al. found that in the absence of CIP, liquid-evolved CIP resistant bacteria were less fit than the ancestor strain and could be easily outcompeted. Notably, similar fitness trade-offs have been reported before and reduced fitness is thought to slow the spread of AMR bacteria. Disturbingly, biofilm-evolved CIP-resistant strains were at least as fit as their ancestor when grown without CIP. Whilst the liquid-evolved strain frequently became resistant to additional antimicrobials, biofilm-grown bacteria usually evolved increased sensitivity to at least one other antimicrobial treatment. This means that although biofilm-evolved strains may be fitter and more likely to spread to new patients, they also may be easier to kill with other antimicrobials.
In this work, Santos-Lopez et al. have focused on how biofilms affect competition and selection pressures during evolution. However, biofilms may alter evolution in other ways and bacterial lifestyle is only one of many factors that shape the evolution of AMR 'in the wild.' For example, many species of bacteria experience increased gene sharing and mutation rates when forming a biofilm, both of which could also drive the evolution of AMR (Steenackers et al., 2016). Additionally, AMR evolution may be driven by increases in mutation rate caused by antimicrobials (Cirz et al., 2005; Kohanski et al., 2010; Gutierrez et al., 2013; Pribis et al., 2019) and other stressors, such as starvation, encountered by bacteria during infection (Fitzgerald and Rosenberg, 2019).
These findings have important implications for treatment of A. baumannii, which is already resistant to multiple drugs and has been deemed a critical threat by both the World Health Organization and the Centers for Disease Control and Prevention in the United States. A better understanding of the variables shaping AMR evolution will improve predictions of evolutionary outcomes, antimicrobial stewardship efforts, and clinical outcomes for individual patients.
References
-
Targeting microbial biofilms: current and prospective therapeutic strategiesNature Reviews Microbiology 15:740–755.https://doi.org/10.1038/nrmicro.2017.99
-
BookTackling Drug-Resitant Infections Globally: Final Report and RecommendationsThe Review on Antimicrobial Resistance, U.K. Department of Health and the Wellcome Trust.
-
Experimental evolution in biofilm populationsFEMS Microbiology Reviews 40:373–397.https://doi.org/10.1093/femsre/fuw002
Article and author information
Author details
Publication history
- Version of Record published: October 25, 2019 (version 1)
Copyright
© 2019, Fitzgerald
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 11,139
- views
-
- 426
- downloads
-
- 9
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Evolutionary Biology
The circadian clock enables anticipation of the day/night cycle in animals ranging from cnidarians to mammals. Circadian rhythms are generated through a transcription-translation feedback loop (TTFL or pacemaker) with CLOCK as a conserved positive factor in animals. However, CLOCK’s functional evolutionary origin and mechanism of action in basal animals are unknown. In the cnidarian Nematostella vectensis, pacemaker gene transcript levels, including NvClk (the Clock ortholog), appear arrhythmic under constant darkness, questioning the role of NvCLK. Utilizing CRISPR/Cas9, we generated a NvClk allele mutant (NvClkΔ), revealing circadian behavior loss under constant dark (DD) or light (LL), while maintaining a 24 hr rhythm under light-dark condition (LD). Transcriptomics analysis revealed distinct rhythmic genes in wild-type (WT) polypsunder LD compared to DD conditions. In LD, NvClkΔ/Δ polyps exhibited comparable numbers of rhythmic genes, but were reduced in DD. Furthermore, under LD, the NvClkΔ/Δ polyps showed alterations in temporal pacemaker gene expression, impacting their potential interactions. Additionally, differential expression of non-rhythmic genes associated with cell division and neuronal differentiation was observed. These findings revealed that a light-responsive pathway can partially compensate for circadian clock disruption, and that the Clock gene has evolved in cnidarians to synchronize rhythmic physiology and behavior with the diel rhythm of the earth’s biosphere.
-
- Computational and Systems Biology
- Evolutionary Biology
A comprehensive census of McrBC systems, among the most common forms of prokaryotic Type IV restriction systems, followed by phylogenetic analysis, reveals their enormous abundance in diverse prokaryotes and a plethora of genomic associations. We focus on a previously uncharacterized branch, which we denote coiled-coil nuclease tandems (CoCoNuTs) for their salient features: the presence of extensive coiled-coil structures and tandem nucleases. The CoCoNuTs alone show extraordinary variety, with three distinct types and multiple subtypes. All CoCoNuTs contain domains predicted to interact with translation system components, such as OB-folds resembling the SmpB protein that binds bacterial transfer-messenger RNA (tmRNA), YTH-like domains that might recognize methylated tmRNA, tRNA, or rRNA, and RNA-binding Hsp70 chaperone homologs, along with RNases, such as HEPN domains, all suggesting that the CoCoNuTs target RNA. Many CoCoNuTs might additionally target DNA, via McrC nuclease homologs. Additional restriction systems, such as Type I RM, BREX, and Druantia Type III, are frequently encoded in the same predicted superoperons. In many of these superoperons, CoCoNuTs are likely regulated by cyclic nucleotides, possibly, RNA fragments with cyclic termini, that bind associated CARF (CRISPR-Associated Rossmann Fold) domains. We hypothesize that the CoCoNuTs, together with the ancillary restriction factors, employ an echeloned defense strategy analogous to that of Type III CRISPR-Cas systems, in which an immune response eliminating virus DNA and/or RNA is launched first, but then, if it fails, an abortive infection response leading to PCD/dormancy via host RNA cleavage takes over.






