UBP12 and UBP13 negatively regulate the activity of the ubiquitin-dependent peptidases DA1, DAR1 and DAR2
Figures
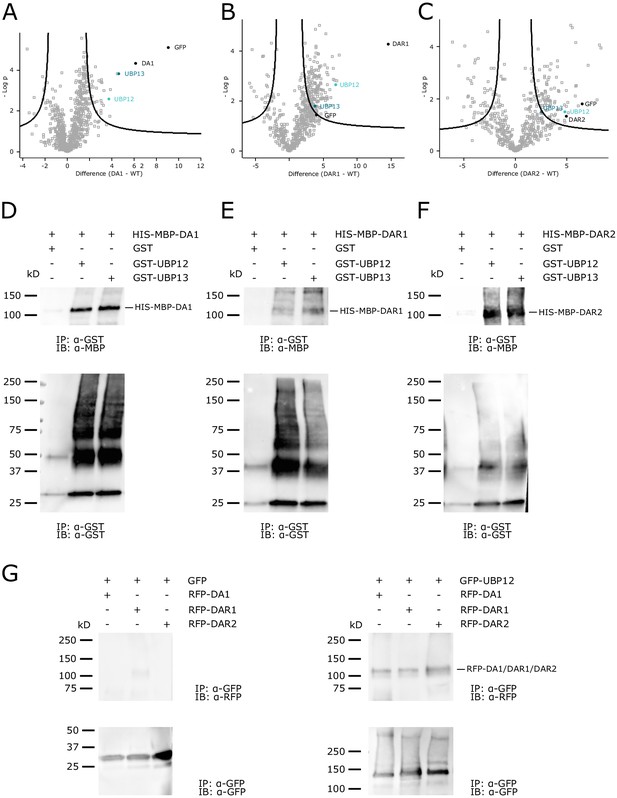
UBP12 and UBP13 interact with DA1, DAR1 and DAR2.
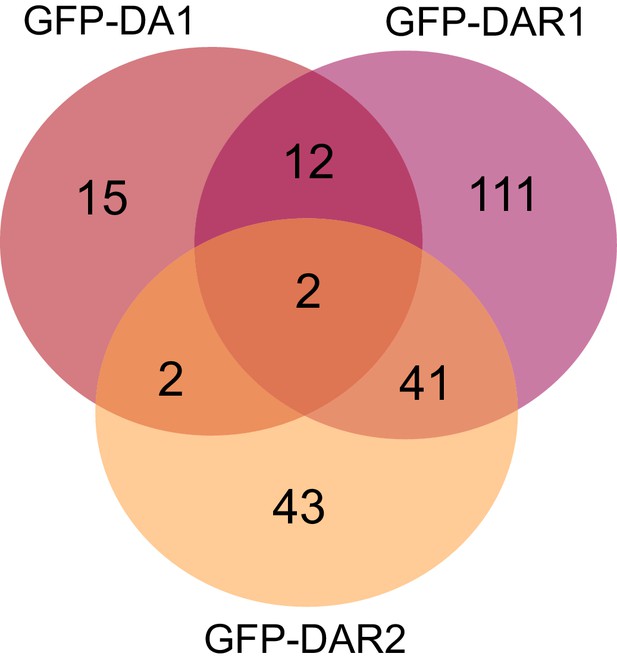
(A–C) Enrichment of the bait, UBP12 and UBP13 compared to the control after immunoprecipitation in (A) 35S::GFP-DA1 (FDR = 0.01, S0 = 1, permutation-based FDR-corrected t-test, Figure 1—source data 1), (B) 35S::GFP-DAR1 (FDR = 0.01, S0 = 1, permutation-based FDR-corrected t-test, Figure 1—source data 1) or (C) 35S::GFP-DAR2 (FDR = 0.05, S0 = 1, permutation-based FDR-corrected t-test, Figure 1—source data 1) seedlings. (D–F) In vitro pull down of (D) HIS-MBP-DA1, (E) HIS-MBP-DAR1 and (F) HIS-MBP-DAR2 with free GST, GST-UBP12 and GST-UBP13. (G) In vivo pull-down of RFP-DA1, RFP-DAR1 and RFP-DAR2 with free GFP and GFP-UBP12.
-
Figure 1—source data 1
List of DA1, DAR1, DAR2 interactors and LFQ intensities by MS/MS.
- https://cdn.elifesciences.org/articles/52276/elife-52276-fig1-data1-v2.xlsx
-
Figure 1—source data 2
MS/MS counts of DA1, DAR1 and DAR2; protein coverage of DA1, DAR1 and DAR2; relative expression levels of DA1, DAR1, DAR2, UBP12 and UBP13 during leaf development.
- https://cdn.elifesciences.org/articles/52276/elife-52276-fig1-data2-v2.xlsx
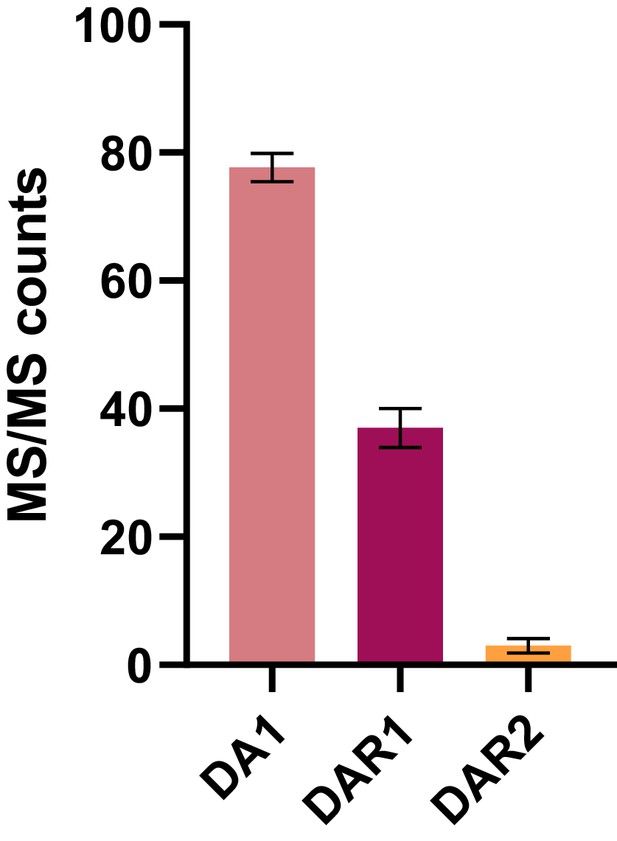
MS/MS counts of DA1, DAR1 and DAR2 baits from GFP-DA1, GFP-DAR1 and GFP-DAR2 pull-downs, respectively (Bars represent the SEM; n = 3 biological repeats; Figure 1—source data 2).
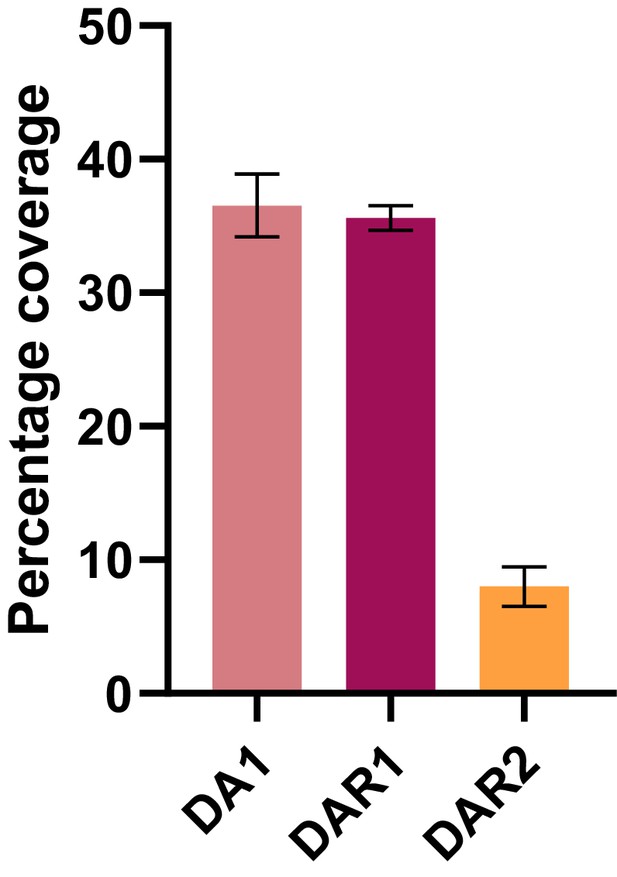
Percentage coverage of the DA1, DAR1 and DAR2 baits from GFP-DA1, GFP-DAR1 and GFP-DAR2 pull-downs, respectively (Bars represent the SEM; n = 3 biological repeats; Figure 1—source data 2).
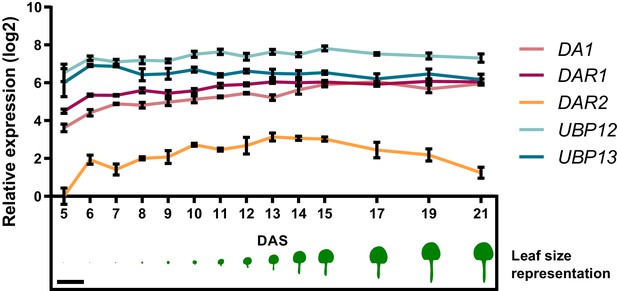
Relative expression levels of UBP12, UBP13, DA1, DAR1 and DAR2 throughout leaf development, scaled to the lowest value (DAR2, 5 DAS; Figure 1—source data 2).
Scale bar represents 1 cm. Bars represent the SEM; n = 3 biological repeats).
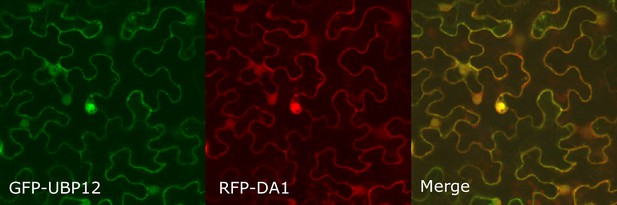
Co-localization of GFP-UBP12 and RFP-DA1 in Nicotiana benthamiana leaves.
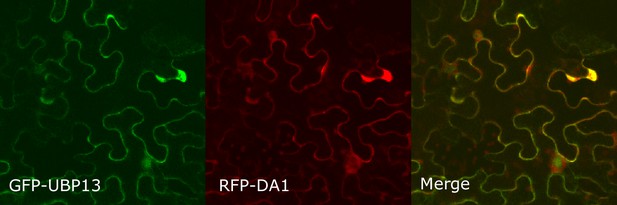
Co-localization of GFP-UBP13 and RFP-DA1 in Nicotiana benthamiana leaves.
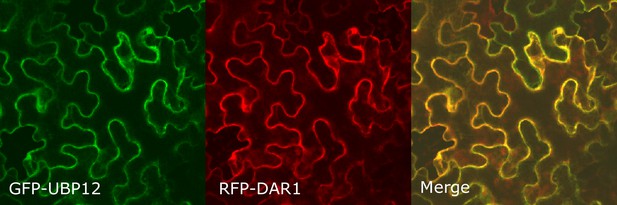
Co-localization of GFP-UBP12 and RFP-DAR1 in Nicotiana benthamiana leaves.
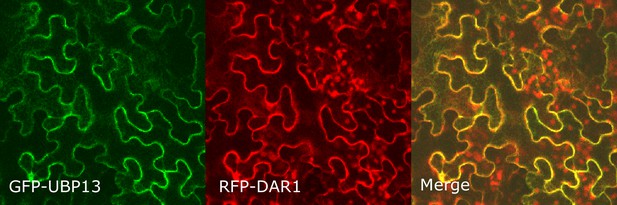
Co-localization of GFP-UBP13 and RFP-DAR1 in Nicotiana benthamiana leaves.
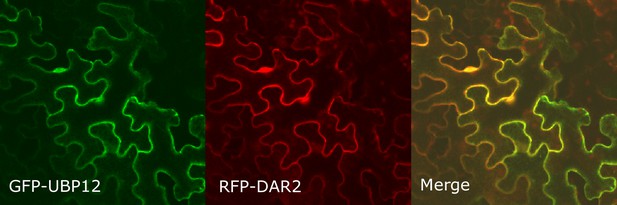
Co-localization of GFP-UBP12 and RFP-DAR2 in Nicotiana benthamiana leaves.
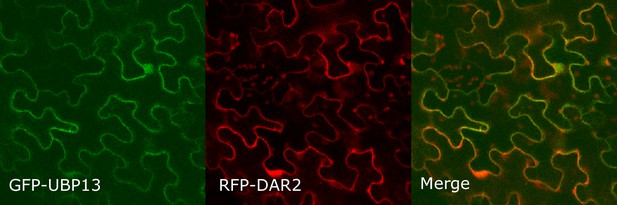
Co-localization of GFP-UBP13 and RFP-DAR2 in Nicotiana benthamiana leaves.
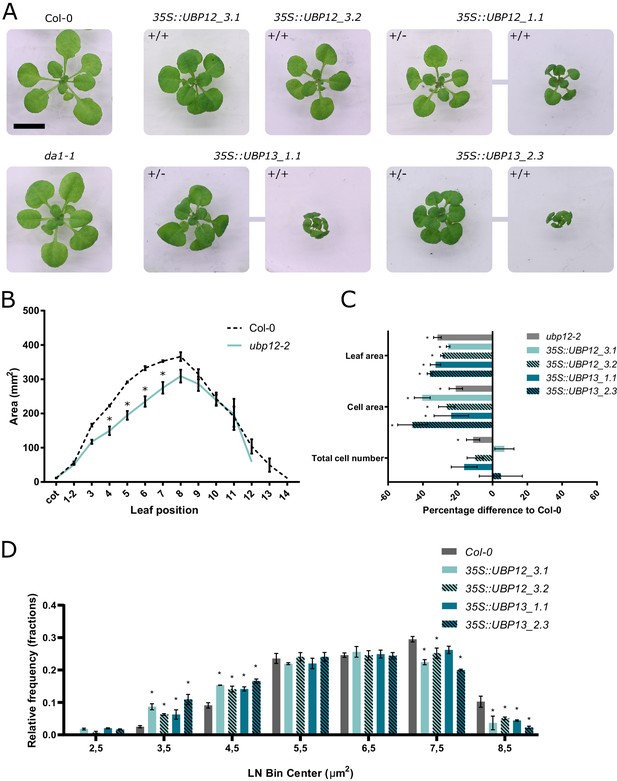
Regulation of leaf size by UBP12 and UBP13.
(A) Twenty-one-day-old plants of Col-0, da1-1 and homozygous (+/+) or heterozygous (+/-) UBP12 and UBP13 overexpression lines. Scale bar represents 1 cm, homozygous and heterozygous plants of the same single locus line are linked in the figure. (B) Leaf area measurements of Col-0 and ubp12-2, n = 3 biological repeats with >10 plants per repeat. (C) Percentage differences of leaf area, cell area and cell number of ubp12-2, 35S::UBP12_3.1, 35S::UBP12_3.2, 35S::UBP13_1.1 and 35S::UBP13_3.2 compared to Col-0, n = 3 biological repeats with three representative leaves per repeat. (D) Relative frequencies of LN transformed cell area distribution of Col-0, 35S::UBP12_3.1, 35S::UBP12_3.2, 35S::UBP13_1.1 and 35S::UBP13_3.2 in 1-µm2 bin sizes, n = 3 biological repeats with three representative leaves per repeat. Bars represent the SEM; * indicates p-value<0.05, ANOVA (Figure 2—source data 1).
-
Figure 2—source data 1
Leaf area analysis and statistics of Col-0 and ubp12-2; leaf area analysis and statistics of Col-0, 35S::UBP12_3.1, 35S::UBP12_3.2, 35S::UBP13_1.1 and 35S::UBP13_3.2; cellular analysis and statistics of Col-0, 35S::UBP12_3.1, 35S::UBP12_3.2, 35S::UBP13_1.1 and 35S::UBP13_3.2; cellular analysis of ubp12-2, relative frequency of the pavement cell area and statistics of Col-0, 35S::UBP12_3.1, 35S::UBP12_3.2, 35S::UBP13_1.1 and 35S::UBP13_3.2.
- https://cdn.elifesciences.org/articles/52276/elife-52276-fig2-data1-v2.xlsx
-
Figure 2—source data 2
Q-RT-PCR data and statistics of UBP12 and UBP13 expression in Col-0, da1-1, 35S::UBP12_3.1, 35S::UBP12_3.2, 35S::UBP13_1.1 and 35S::UBP13_3.2 overexpression lines; leaf area data and statistics of ubp12-1; leaf area data and statistics of ubp13-1; leaf area data and statistics of ubp13-2; leaf area data and statistics of ubp13-3; relative frequency of ubp12-2 pavement cell area data and statistics.
- https://cdn.elifesciences.org/articles/52276/elife-52276-fig2-data2-v2.xlsx
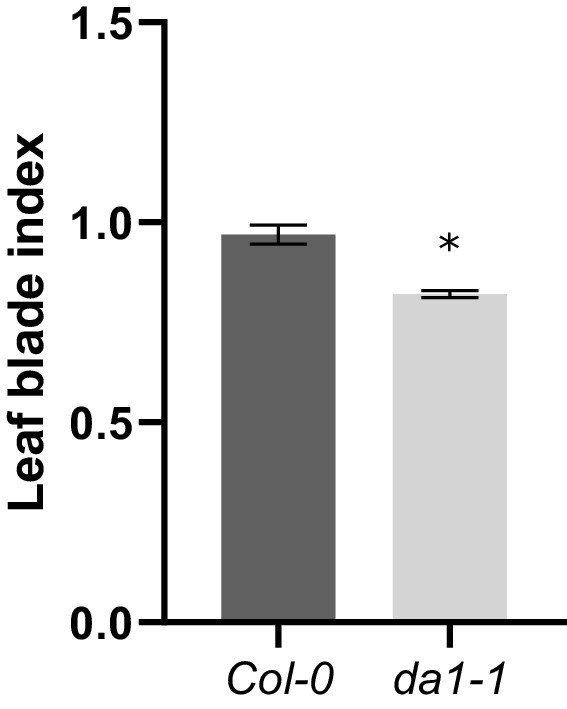
Leaf index measurements of Col-0 and da1-1 mutants, n = 3 biological repeats with >6 leaves per repeat.
Bars represent the SEM; * indicates p-value<0.05, two-tailed t-test; (Figure 2—source data 2).
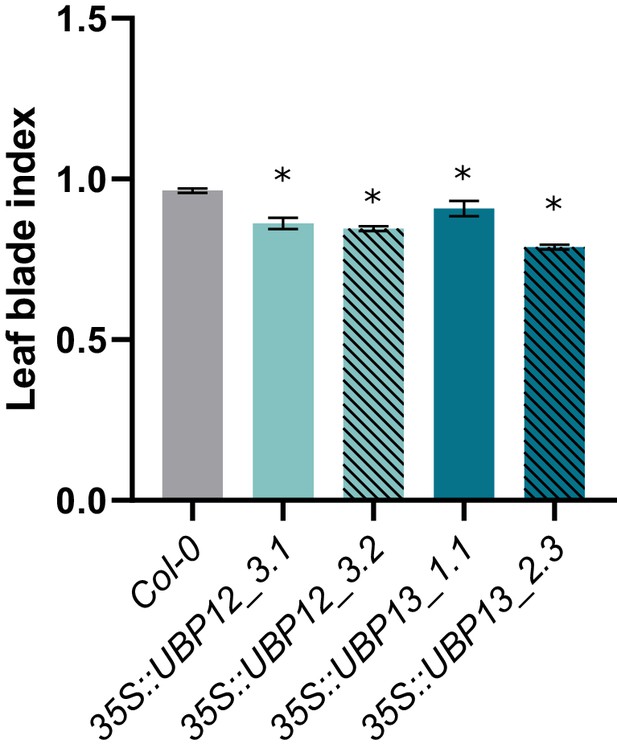
Leaf index measurements of Col-0, 35S::UBP12_3.1, 35S::UBP12_3.2, 35S::UBP13_1.1 and 35S::UBP13_3.2 overexpression lines.
Bars represent the SEM; * indicates p-value<0.05, ANOVA; (Figure 2—source data 2).
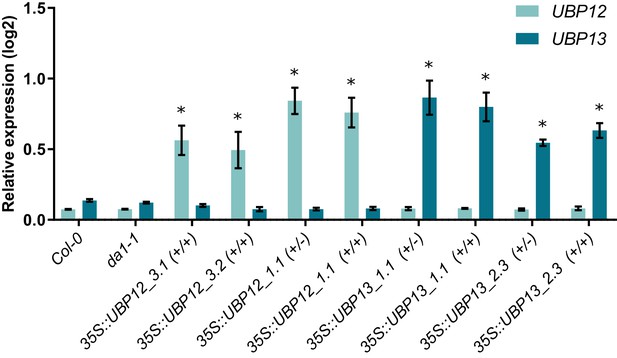
UBP12 and UBP13 transcript levels in Col-0, da1-1, 35S::UBP12_3.1, 35S::UBP12_3.2, 35S::UBP13_1.1 and 35S::UBP13_3.2 overexpression lines, n = 3 biological repeats with >8 leaves per repeat.
Bars represent the SEM; * indicates p-value<0.05, ANOVA; (Figure 2—source data 2).
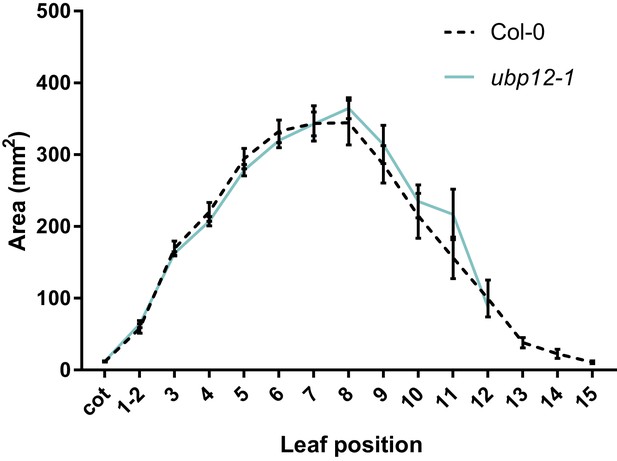
Leaf area measurements of ubp12-1, compared to Col-0, n = 3 biological repeats with >10 plants per repeat.
Bars represent the SEM; * indicates p-value<0.05, ANOVA; (Figure 2—source data 2).
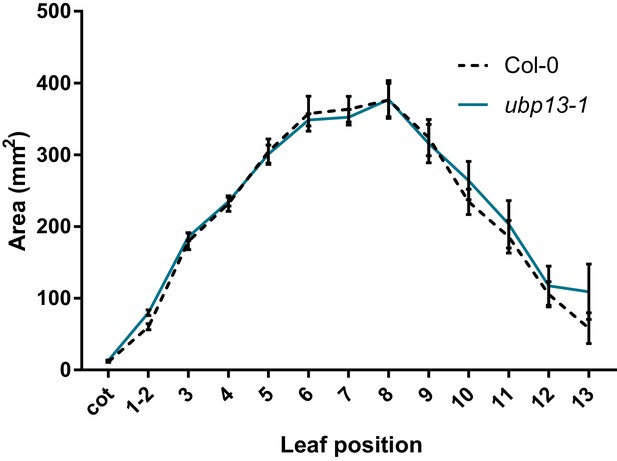
Leaf area measurements of ubp13-1, compared to Col-0, n = 3 biological repeats with >10 plants per repeat.
Bars represent the SEM; * indicates p-value<0.05, ANOVA; (Figure 2—source data 2).
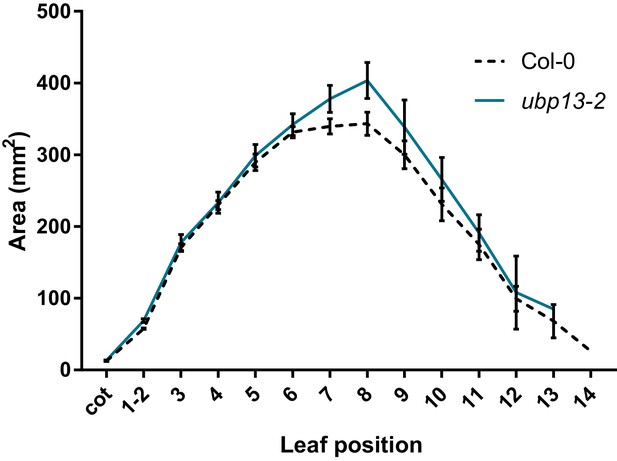
Leaf area measurements of ubp13-2 and compared to Col-0, n = 3 biological repeats with >10 plants per repeat.
Bars represent the SEM; * indicates p-value<0.05, ANOVA; (Figure 2—source data 2).
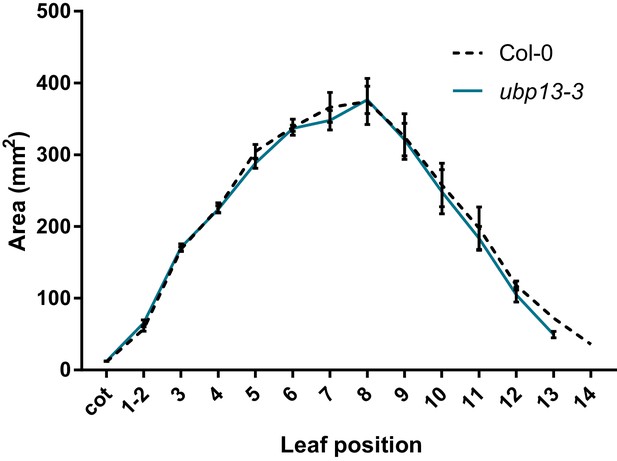
Leaf area measurements of ubp13-3 compared to Col-0, n = 3 biological repeats with >10 plants per repeat.
Bars represent the SEM; * indicates p-value<0.05, ANOVA; (Figure 2—source data 2).
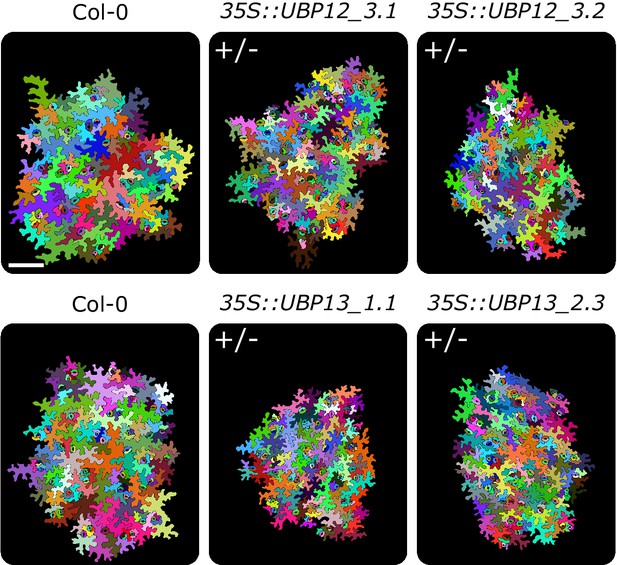
Representations of abaxial leaf epidermal cells of Col-0, homozygous 35S::UBP12_3.1, homozygous 35S::UBP12_3.2, heterozygous 35S::UBP13_1.1 and heterozygous 35S::UBP13_3.2.
(+/-) and (+/+) indicate heterozygous and homozygous plants, respectively, scale-bar represents 0.1 mm.
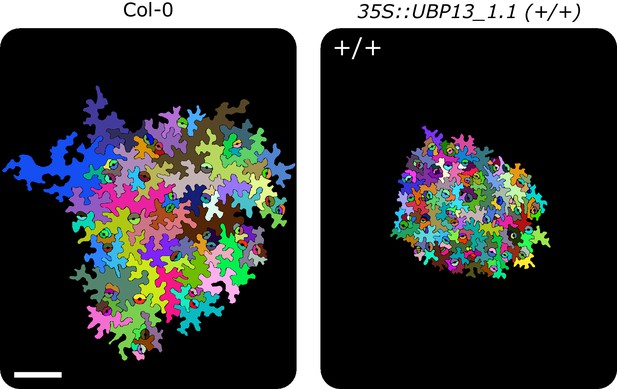
Representations of abaxial leaf epidermal cells of Col-0 and the homozygous 35S::UBP13_1.1, (+/+) indicates homozygous plants, scale-bar represents 0.1 mm.
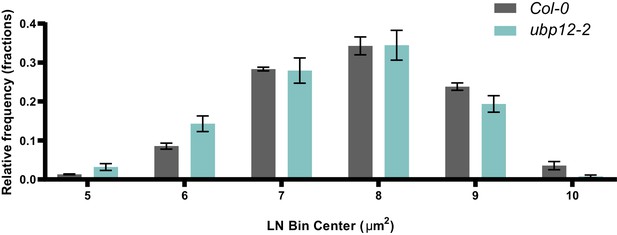
Cell size distribution plot of Col-0 and ubp12-2, n = 3 biological repeats with three representative leaves per repeat.
Bars represent the SEM; * indicates p-value<0.05, ANOVA; (Figure 2—source data 2).
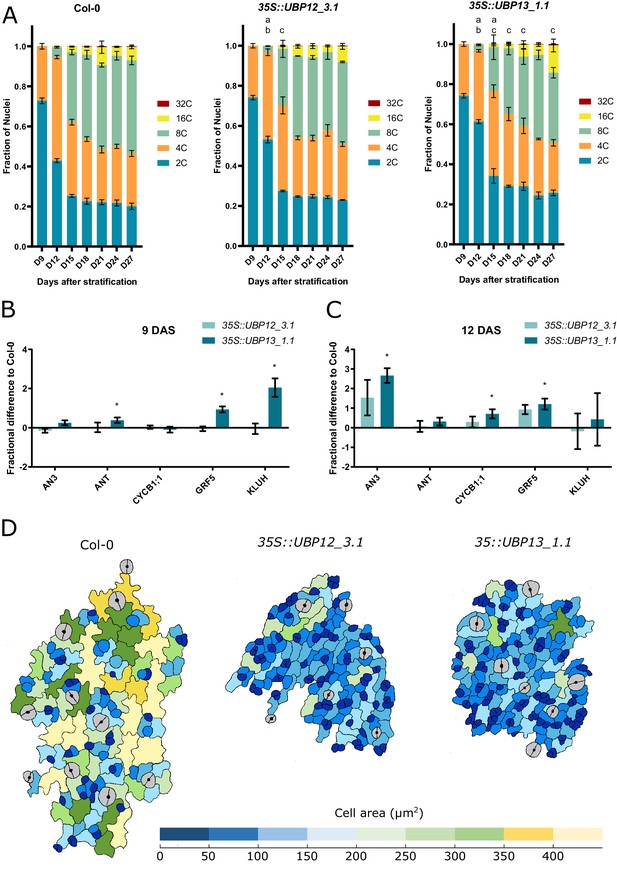
UBP12 and UBP13 regulate the onset of endoreduplication.
(A) Ploidy distribution of nuclear DNA in Col-0, 35S::UBP12_3.1 and 35S::UBP13_1.1. (B–C) Fractional difference in expression of cell proliferation markers in 35S::UBP12_3.1 and 35S::UBP13_1.1 compared to Col-0 at (B) nine and (C) 12 DAS. (D) Representations of abaxial leaf epidermal cells at the tip of the third leaf of Col-0, 35S::UBP12_3.1 and 35S::UBP13_1.1 at 12 DAS. Bars represent the SEM, n = 3 biological repeats with >3 leaves per repeat; a, b and c indicate a significant difference in 2C, 4C and 8C, respectively; * indicates p-value<0.05, ANOVA, (Figure 3—source data 1).
-
Figure 3—source data 1
Flow cytometry counts and statistics; Q-RT-PCR data and statistics of proliferation markers in developing leaves (9 DAS and 12 DAS).
- https://cdn.elifesciences.org/articles/52276/elife-52276-fig3-data1-v2.xlsx
-
Figure 3—source data 2
Endoreduplication index calculations and statistics; Q-RT-PCR data and statistics of proliferation markers in developing leaves (15 DAS and 18 DAS).
- https://cdn.elifesciences.org/articles/52276/elife-52276-fig3-data2-v2.xlsx
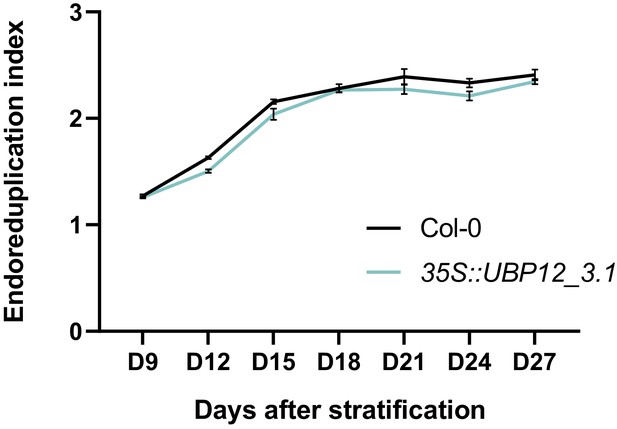
Endoreduplication index of 35S::UBP12_3.1 leaf nuclei compared to Col-0.
Bars represent the SEM; n = 3 biological repeats with >3 leaves per repeat; * indicates p-value<0.05, ANOVA, (Figure 3—source data 2).
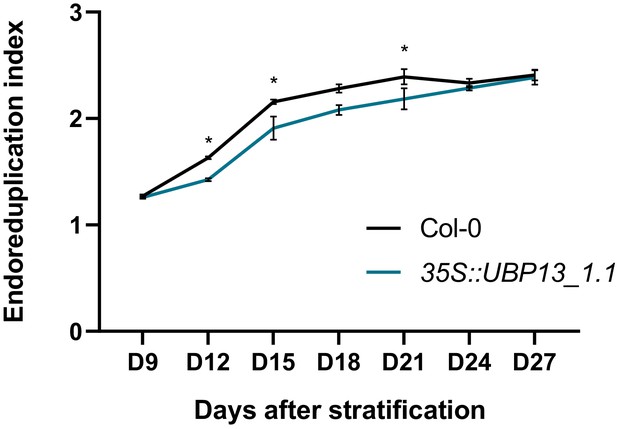
Endoreduplication index of 35S::UBP13_1.1 leaf nuclei compared to Col-0.
Bars represent the SEM; n = 3 biological repeats with >3 leaves per repeat; * indicates p-value<0.05, ANOVA, (Figure 3—source data 2).
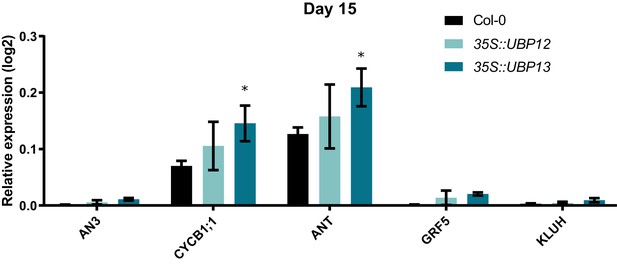
Relative expression levels of cell proliferation markers in Col-0, 35S::UBP12_3.1 and 35S::UBP13_1.1 at 15 DAS.
Bars represent the SEM; n = 3 biological repeats with >3 leaves per repeat; * indicates p-value<0.05, ANOVA, (Figure 3—source data 2).
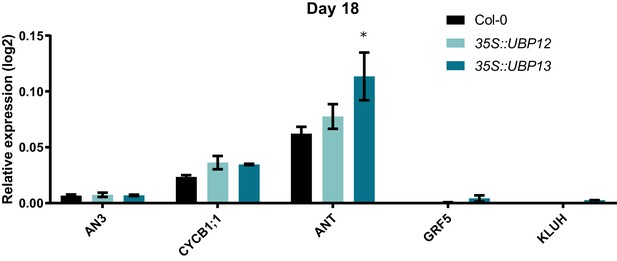
Relative expression levels of cell proliferation markers in Col-0, 35S::UBP12_3.1 and 35S::UBP13_1.1 at 18 DAS.
Bars represent the SEM; n = 3 biological repeats with >3 leaves per repeat; * indicates p-value<0.05, ANOVA, (Figure 3—source data 2).
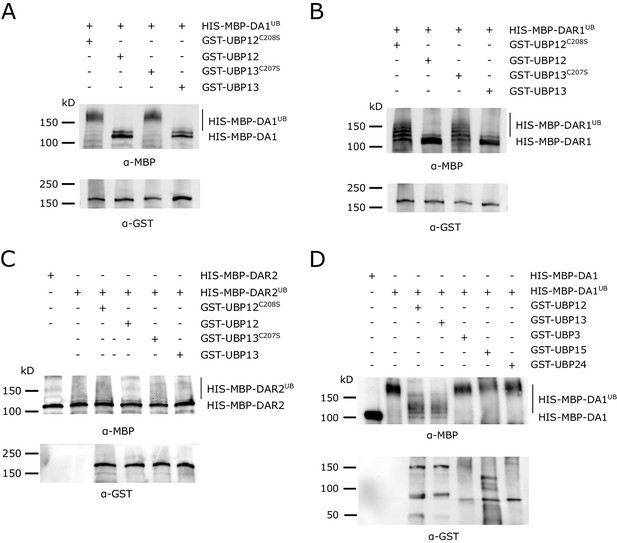
In vitro deubiquitination specificity of DA1, DAR1 and DAR2 by UBP12 and UBP13.
(A–C) In vitro deubiquitination assays with GST-UBP12, GST-UBP12C208S, GST-UBP13 or GST-UBP13C207S of (A) HIS-MBP-DA1, (B) HIS-MBP-DAR1 and (C) HIS-MBP-DAR2. (D) Deubiquitination assay with GST-UBP12, GST-UBP13, GST-UBP3, GST-UBP15 and GST-UBP24 of HIS-MBP-DA1.
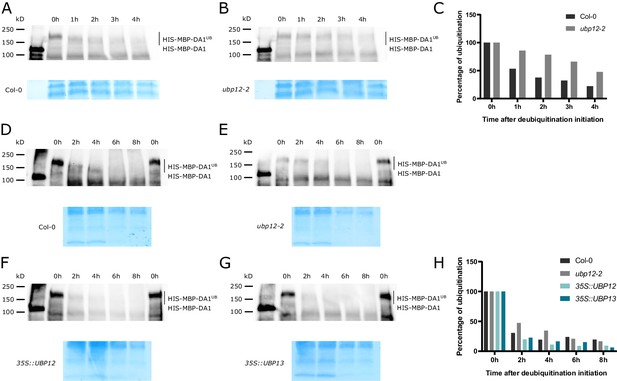
Deubiquitination of DA1, DAR1 and DAR2 by UBP12 and UBP13 in vivo.
(A–B) Cell-free deubiquitination assay of ubiquitinated HIS-MBP-DA1 proteins incubated with (A) Col-0 or (B) ubp12-2 extract. (C) Quantification of deubiquitination (Figure 5—source data 1). (D–G) Cell-free deubiquitination assay of ubiquitinated HIS-MBP-DA1 with (D) Col-0, (E) ubp12-2, (F) 35S::UBP12 or (G) 35S::UBP13 extracts. (H) Quantification of deubiquitination (Figure 5—source data 1).
-
Figure 5—source data 1
Calculation of in vivo deubiquitination.
- https://cdn.elifesciences.org/articles/52276/elife-52276-fig5-data1-v2.xlsx
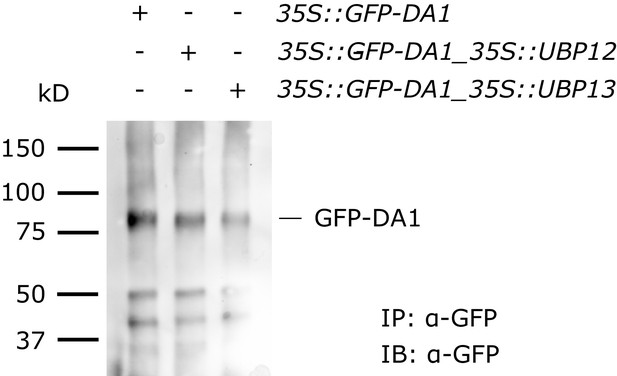
Stability of DA1 in 35::GFP-DA1, 35::GFP-DA1_35::UBP12 and 35S::GFP-DA1_35S::UBP13 seedlings.
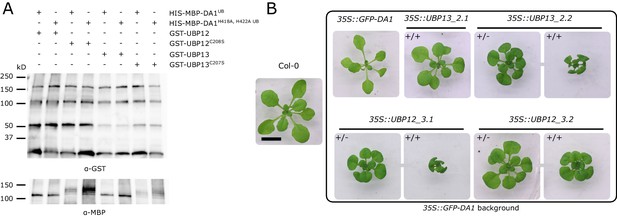
Cleaving assay on UBP12 and UBP13.
(A) In vitro cleaving assay on GST-UBP12, GST-UBP12C208S, GST-UBP13 and GST-UBP13C207S by HIS-MBP-DA1 and the peptidase-deficient HIS-MBP-DA1H418A,H422A. (B) Twenty-one-day-old plants of Col-0, 35S::GFP-DA1, 35S::UBP12_3.1/35S::GFP-DA1, 35S::UBP12_3.2/35S::GFP-DA1, 35S::UBP13_2.1/35S::GFP-DA1 and 35S::UBP13_2.2/35S::GFP-DA1 homozygous (+/+) and heterozygous (+/-) double overexpression lines. Scale bar represents 1 cm.
-
Figure 6—source data 1
Q-RT-PCR data and statistics of DA1, UBP12 and UBP13 expression in Col-0, 35S::GFP-DA1, 35S::UBP12_3.1/35S::GFP-DA1, 35S::UBP12_3.2/35S::GFP-DA1, 35S::UBP13_2.1/35S::GFP-DA1 and 35S::UBP13_2.2/35S::GFP-DA1.
- https://cdn.elifesciences.org/articles/52276/elife-52276-fig6-data1-v2.xlsx
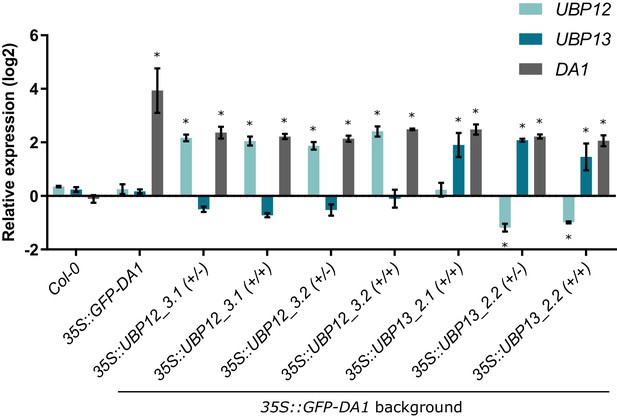
Relative expression levels of DA1, UBP12 and UBP13 in the double 35S::GFP-DA1_35S::UBP12 and 35S::GFP-DA1_35S::UBP13 overexpression lines.
n = 3 biological repeats with >3 leaves per repeat, * indicates p-value<0.05 compared to the expression levels in Col-0, ANOVA (Figure 6—source data 1).
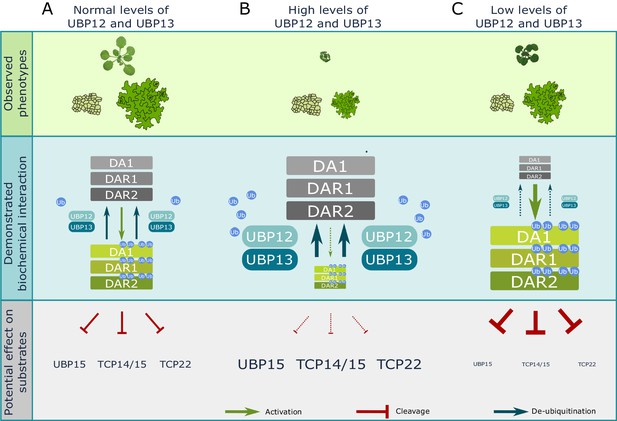
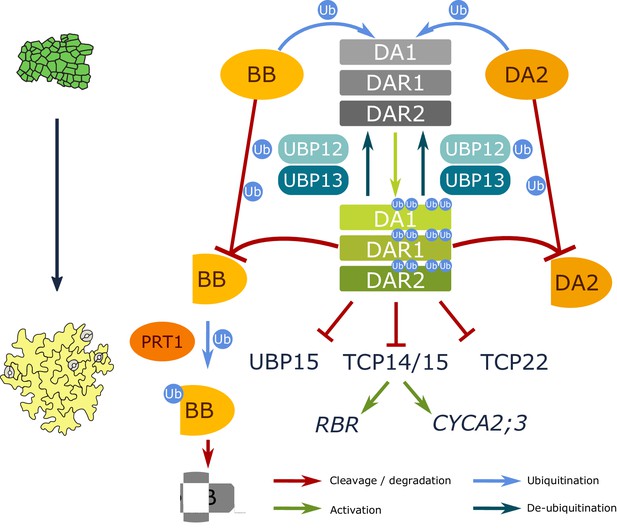
Model of UBP12 and UBP13 levels on leaf area and cellular phenotypes.
Molecular balance and leaf phenotypes in (A) wild-type conditions, (B) high UBP12 and UBP13 expression lines and (C) lower levels of UBP12 and UBP13.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Arabidopsis thaliana) | DA1 | TAIR | AT1G19270 | |
| Gene (Arabidopsis thaliana) | DAR1 | TAIR | AT4G36860 | |
| Gene (Arabidopsis thaliana) | DAR2 | TAIR | AT2G39830 | |
| Gene (Arabidopsis thaliana) | UBP12 | TAIR | AT5G06600 | |
| Gene (Arabidopsis thaliana) | UBP13 | TAIR | AT3G11910 | |
| Gene (Arabidopsis thaliana) | UBP3 | TAIR | AT4G39910 | |
| Gene (Arabidopsis thaliana) | UBP15 | TAIR | AT1G17110 | |
| Gene (Arabidopsis thaliana) | UBP24 | TAIR | AT4G30890 | |
| Gene (Arabidopsis thaliana) | DA2 | TAIR | AT1G78420 | |
| Cell line (Arabidopsis thaliana) | Landsberg erecta | TAIR/ABRC | Germplasm:6530492727 NASC stock number: N84840 | https://www.arabidopsis.org/servlet/TairObject?id=4502009498&type=stock |
| Strain, strain background (Escherichia coli) | DH5 α | Thermo-fisher | 18258012 | Chemically competent cells |
| Strain, strain background (Escherichia coli) | BL21(DE3) | Thermo-fisher | EC0114 | Chemically competent cells |
| 35S::RFP-DA1 | 35S::DA2-FLAG | p35S::GFP-UBP12 | pUBQ10::HA-BB | Cleaving inhibition |
|---|---|---|---|---|
| 35S::RFP-DA1 | 35S::DA2-FLAG | p35S::GFP-UBP12C208S | pUBQ10::HA-BB | Cleaving |
| 35S::RFP-DA1 | 35S::DA2-FLAG | p35S::GFP-UBP13 | pUBQ10::HA-BB | Cleaving inhibition |
| 35S::RFP-DA1 | 35S::DA2-FLAG | p35S::GFP-UBP13C207S | pUBQ10::HA-BB | Cleaving |
| 35S::HA-DA1 | 35S::DA2-FLAG | pUBP10::UBP12-BFP | pUBP10::NLS-GFP-TCP14-mCherry | Cleaving inhibition, FRET |
|---|---|---|---|---|
| 35S::HA-DA1 | 35S::DA2-FLAG | pUBP10::UBP12C208S–BFP | pUBP10::NLS-GFP-TCP14-mCherry | Cleaving, loss of FRET |
| 35S::HA-DA1 | 35S::DA2-FLAG | pUBP10::UBP13-BFP | pUBP10::NLS-GFP-TCP14-mCherry | Cleaving inhibition, FRET |
| 35S::HA-DA1 | 35S::DA2-FLAG | pUBP10::UBP13C208S–BFP | pUBP10::NLS-GFP-TCP14-mCherry | Cleaving, loss of FRET |
Additional files
-
Supplementary file 1
Primer list.
- https://cdn.elifesciences.org/articles/52276/elife-52276-supp1-v2.xlsx
-
Supplementary file 2
Conditions recombinant protein production.
- https://cdn.elifesciences.org/articles/52276/elife-52276-supp2-v2.xlsx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/52276/elife-52276-transrepform-v2.docx