A moth odorant receptor highly expressed in the ovipositor is involved in detecting host-plant volatiles
Figures
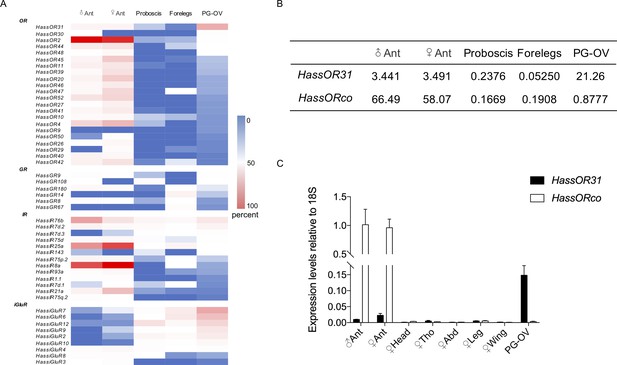
Gene expression of HassOR31 in different tissues of H. assulta.
(A) Tissue expression profiles of putative chemosensory receptor genes identified in the pheromone gland-ovipositor complex (PG-OV) of H. assulta. (B) TPM values of HassOR31 and HassORco in different tissues. Ant, antennae; Proboscis, mixed female and male proboscis; Forelegs, mixed female and male forelegs. (C) qRT-PCR results of HassOR31 and HassORco in different tissues relative to housekeeping gene 18S. Tho, thorax; Abd, abdomen.
-
Figure 1—source data 1
Source data for Figure 1A and C.
- https://cdn.elifesciences.org/articles/53706/elife-53706-fig1-data1-v2.xlsx

Tissue expression profiles of some putative chemosensory related genes identified in the pheromone gland-ovipositor complex of H. assulta.
TPM values are listed below different tissues. OBP, odorant binding protein; CSP, chemosensory protein; GOBP, general odorant binding protein; PBP, pheromone binding protein; SNMP, sensory neuron membrane protein.
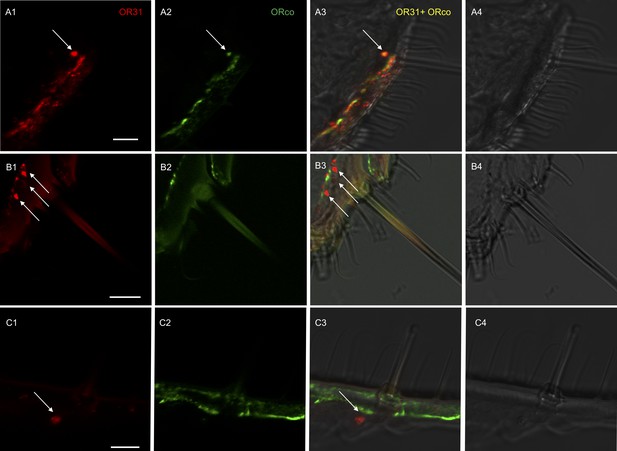
Localization of HassOR31 and HassORco expression in cells of H. assulta ovipositors.
Double-FISH with female ovipositors using combinations of labeled OR probes and visualization of cells bearing distinct HassOR31 transcripts by red (DIG) (A1, B1, C1) and HassORco transcripts by green (biotin) (A2, B2, C2) fluorescence, respectively. Co-labelling of cells by both OR probes appear as yellow/orange color in the overlay of the red and green fluorescence channels (A3, B3, C3). Bright-field images are presented as references (A4, B4, C4). Arrows indicate the cell location. Scale bars: 10 µm. (A1-3) The HassOR31 and HassORco probes label the same cell. (B1-3, C1-3) Only the HassOR31 probe is detected. Scale bars: 10 μm.
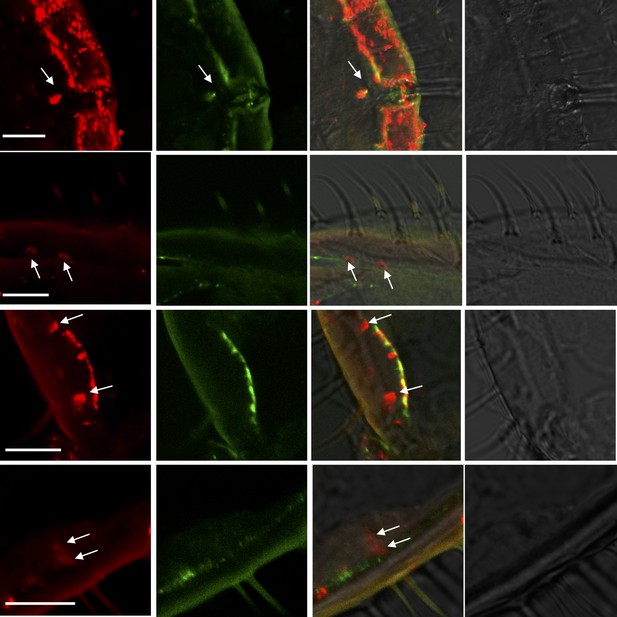
More examples of two-color in situ hybridization visualizing the combinations of HassOR31 (red) and HassORco (green) in the ovipositor of H. assulta.
Arrows indicate the cell location. Scale bars: 10 μm.
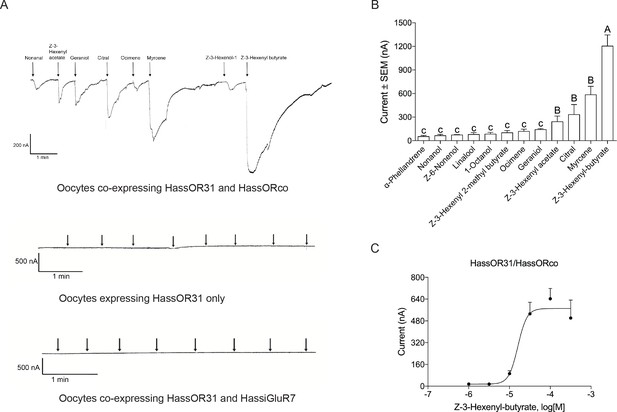
Functional analyses of chemosensory receptors in Xenopus oocytes.
(A) Inward current responses of Xenopus oocytes with expressed HassOR31/HassORco, HassOR31 alone, or HassOR31/HassiGluR7 in response to odorants (10−4 M solution). Odorants were applied for 8 s at times indicated by arrowheads; (B) Odorant-response spectra of Xenopus oocytes with expressed HassOR31/HassORco. Responses were measured as induced inward currents, expressed in nA. Error bars show standard error of the mean (n = 3–5), columns with different letters are significantly different at p<0.05 (One-way ANOVA followed by post hoc analysis with Turkey test); (C) Dose responses of Xenopus oocytes with expressed HassOR31/HassORco to the most effective ligand Z-3-hexenyl butyrate (n = 4), The EC50 value for Z-3-hexenyl butyrate was 1.606 × 10–5 M.
-
Figure 3—source data 1
Source data for Figure 3B and C.
- https://cdn.elifesciences.org/articles/53706/elife-53706-fig3-data1-v2.xlsx
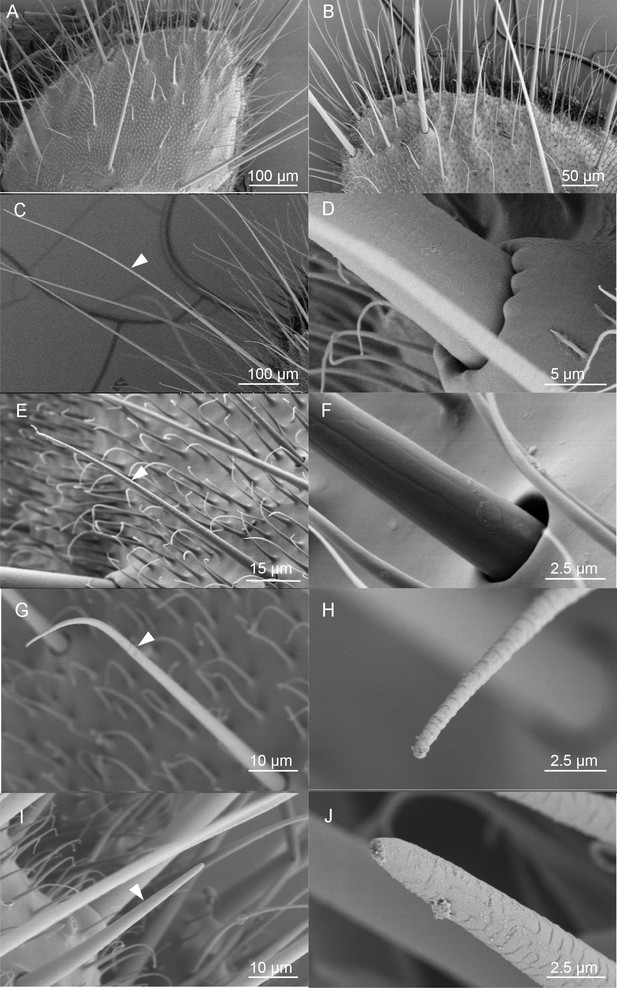
Scanning electron micrographs (SEM) of the ovipositor of H. assulta and its associated sensilla.
(A) An overview of the extended tip of the ovipositor of H. assulta, which contains two anal papillae surrounding the ovipore. (B) Details of the papilla close to the ovipore. There are four types of sensilla distributed on each papilla: Type I (C), putative mechanical sensilla widely distributed on the papilla, with raised pocket-like bases and a smooth surface (D); Type II (E), putative mechanical sensilla with sockets, showing flexible areas and non-porous surfaces (F); Type III (G), putative olfactory/taste sensilla shorter than Type I sensilla and morphologically similar to the trichoid on antennae, with pores on both the surface and the tip (H); Type IV (I), putative olfactory/taste sensilla located near the ovipore and morphologically similar to the basiconic sensilla, with pores on the surface and a large terminal pore (J).
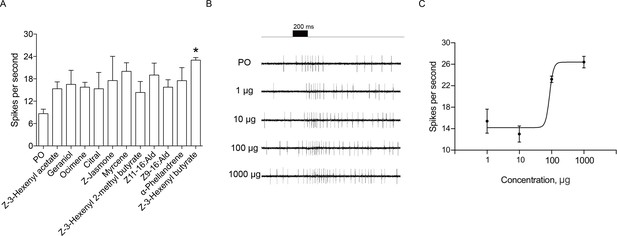
Single sensillum recordings (SSR) of putative chemosensory sensilla in an H. assulta ovipositor.
(A) The firing rate of a putative chemosensory sensilla to several plant volatile and sex pheromone components. Chemicals with * induced significantly electrophysiological responses at p<0.05 relative to PO (paraffin oil) (One-way ANOVA followed by post hoc analysis with Turkey test, n = 3–4; Z-3-Hexenyl butyrate, p=0.0224). (B) The exemplary recordings of electrophysiological activities in a putative chemosensory sensilla to different load doses (1 µg, 10 µg, 100 µg and 1000 µg) of Z-3-hexenyl butyrate. (C) SSR dose responses of putative chemosensory sensilla to Z-3-hexenyl butyrate (n = 5), EC50 = 86.50 μg.
-
Figure 5—source data 1
Source data for Figure 5A and C.
- https://cdn.elifesciences.org/articles/53706/elife-53706-fig5-data1-v2.xlsx
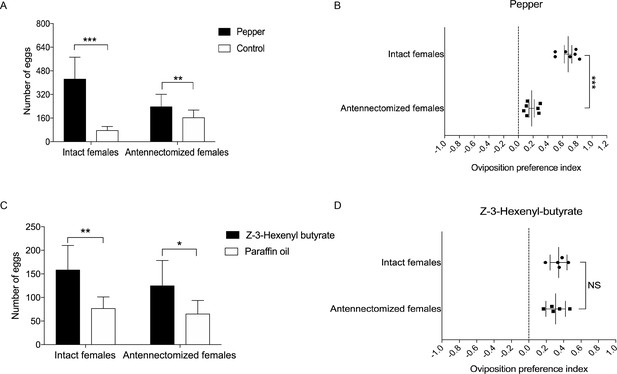
Oviposition preference of the antennae amputated and intact mated females of H. assulta.
(A) Number of eggs on the hot pepper treated gauze and control gauze. Two and three asterisks indicate significant difference (p<0.01 and p<0.001, paired t test). (B) Oviposition preference index between the hot pepper treated gauze and control gauze. Three asterisks indicate significant difference (p<0.001, unpaired t test). (C) Number of eggs on the Z-3-hexenyl butyrate treated fake leaves and control fake leaves. One and two asterisks indicate significant difference (p<0.05 and p<0.01, paired t test). (D) The oviposition preference index between the Z-3-hexenyl butyrate treated fake leaves and control fake leaves. NS indicates no significant difference (p>0.05, unpaired t test). All experiments were carried out with five to seven biological replications, with 10–15 mated female moths used in each replicate.
-
Figure 6—source data 1
Source data for Figure 6A, B, C and D.
- https://cdn.elifesciences.org/articles/53706/elife-53706-fig6-data1-v2.xlsx

The set-up of oviposition choice tests and the spread of eggs laid by mated females of H. assulta.
(A) The choice test 1, the pepper treated gauzes vs control gauzes. The gauze was equally divided into four sections. The diameter of the hot pepper fruit discs is 1.5 cm. To avoid the moths directly contacting the hot pepper fruit discs, a stainless-steel cloth was put on the gauze. (B) The choice test 2, Z-3-hexenyl butyrate treated fake plants vs paraffin oil treated fake plants. Z-3-Hexenyl butyrate dissolved paraffin oil or paraffin oil were dropped into rubber heads, and one rubber head was placed on the leaf of one fake plant. (C and D) The spread of eggs laid by intact and antennae amputated mated females in the choice test 1, respectively. Eggs were marked in red.
Videos
Z-stack video of two-color in situ hybridization visualizing the combinations of HassOR31 (red) and HassORco (green) in the ovipositor of H. assulta.
White arrows indicate the cells only expressing HassOR31, and yellow arrow the cell co-expressing HassOR31 and HassORco.
An exemplary SSR experiment video.
Tables
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Gene (Helicoverpa assulta) | 18S ribosomal RNA gene | NCBI | GenBank: EU057177.1 | |
| Commercial assay, kit | RNeasy Plus Universal Mini Kit | Qiagen | Cat# 73404 | |
| Commercial assay, kit | Dynabeads mRNA purification kit | Invitrogen | Cat# 61006 | |
| Commercial assay, kit | Q5 High-Fidelity DNA Polymerase | NEB | Cat# M0491 | |
| Commercial assay, kit | M-MLV reverse transcriptase | Promega | Cat# M1701 | |
| Commercial assay, kit | SYBR Premix Ex TaqII | Takara | Cat# RR820 | |
| Commercial assay, kit | T7/SP6 RNA transcription system | Roche | Cat# 10999644001 | |
| Commercial assay, kit | mMESSAGE mMACHINE SP6 | Ambion | Cat# AM1340 | |
| Software, algorithm | Trimmomatic | Trimmomatic | RRID:SCR_011848 | |
| Software, algorithm | FastQC | FastQC | RRID:SCR_014583 | |
| Software, algorithm | Trinity | Trinity | RRID:SCR_013048 | |
| Software, algorithm | RSEM | RSEM | RRID:SCR_013027 | |
| Software, algorithm | GraphPad Prism | GraphPad Prism | RRID:SCR_002798 | 7.0 |
| Software, algorithm | ZEN Digital Imaging for Light Microscopy | ZEN Digital Imaging for Light Microscopy | RRID:SCR_013672 | 2012 |
| Software, algorithm | Adobe Illustrator | Adobe systems | RRID:SCR_014198 | CS6 |
| Software, algorithm | pCLAMP software | pCLAMP software | RRID:SCR_011323 |
Additional files
-
Supplementary file 1
Expression values of putative chemosensory receptors in the pheromone gland-ovipositor complex of H. assulta (Hass) and H. armigera (Harm).
OR, odorant receptor; GR, gustatory receptor; IR, antennal ionotropic receptor; iGluR, ionotropic glutamate receptor.
- https://cdn.elifesciences.org/articles/53706/elife-53706-supp1-v2.docx
-
Supplementary file 2
Primers used for qRT-PCR, RT-PCR, probe synthesis and full-length cDNA cloning.
- https://cdn.elifesciences.org/articles/53706/elife-53706-supp2-v2.docx
-
Supplementary file 3
Tested compounds for functional analysis of HassOR31.
- https://cdn.elifesciences.org/articles/53706/elife-53706-supp3-v2.docx
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/53706/elife-53706-transrepform-v2.docx





