Ciliate mitoribosome illuminates evolutionary steps of mitochondrial translation
Figures
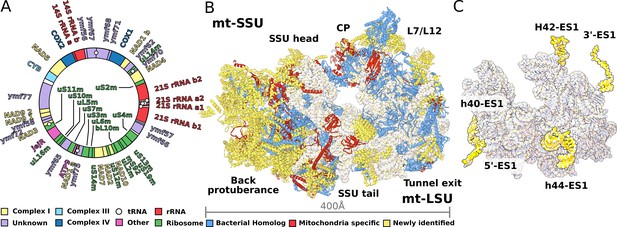
Structure of T. thermophila mitoribosome and newly identified proteins in the mitochondrial DNA.
(A) Schematic representation of the mitochondrial genome of T. thermophila with newly identified proteins labeled inside the circle. (B) The overall structure of the mitoribosome showing mitochondria specific and newly identified proteins. (C) Mitoribosomal rRNA showing expansion segments (relative to E. coli) in yellow.
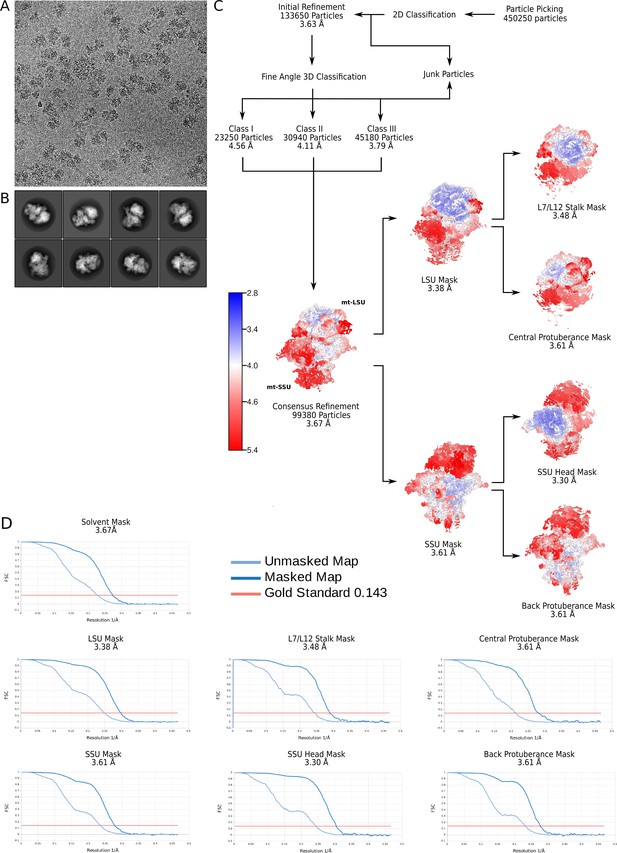
Electron microscopy data processing workflow.
(A) Representative electron micrograph at 1.07 Å2/px. (B) 2D classes showing views of the T. thermophila mitoribosome. (C) Data processing steps with the final maps colored by local resolution. (D) Gold-standard Fourier shell correlation (FSC) curves for the final maps.
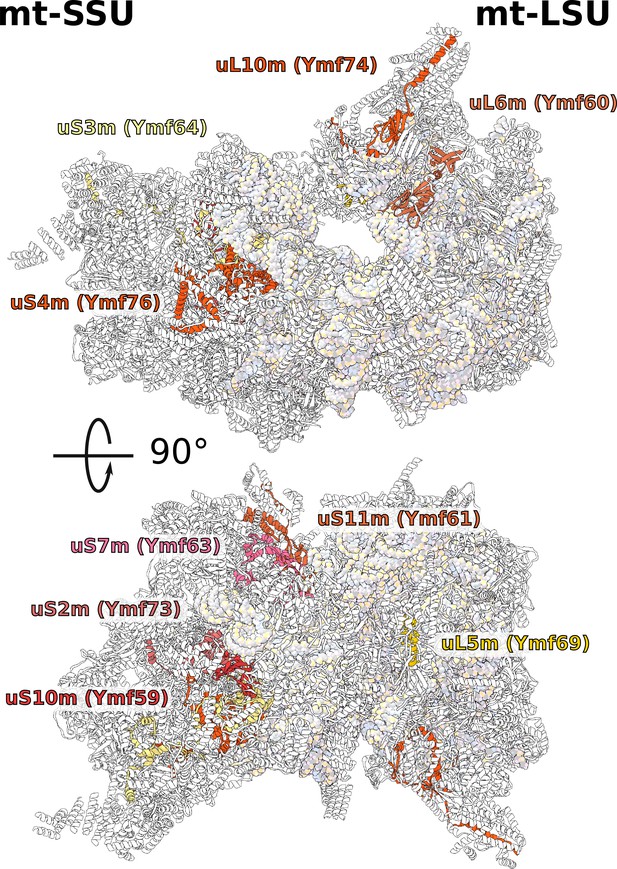
Positions of newly annotated proteins on the mitoribosome.
Nine proteins with previously unknown function, which were named Ymf, have been identified as mitoribosomal proteins and modeled as shown.
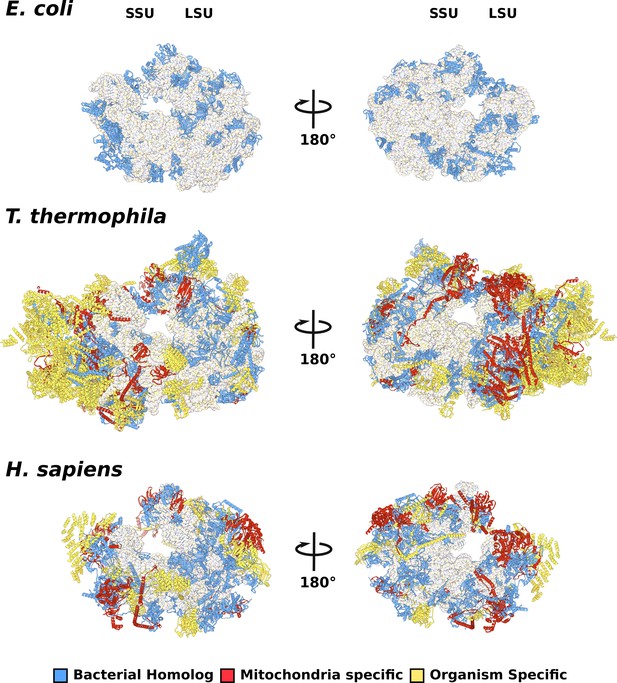
Comparison of evolutionary conservation for mitoribosomal proteins.
The overall structures of E. coli (PDBID: 5MDZ), T. thermophila (PDBID: 6Z1P), and H. sapiens (PDBID: 3j9m) colored by protein conservation.
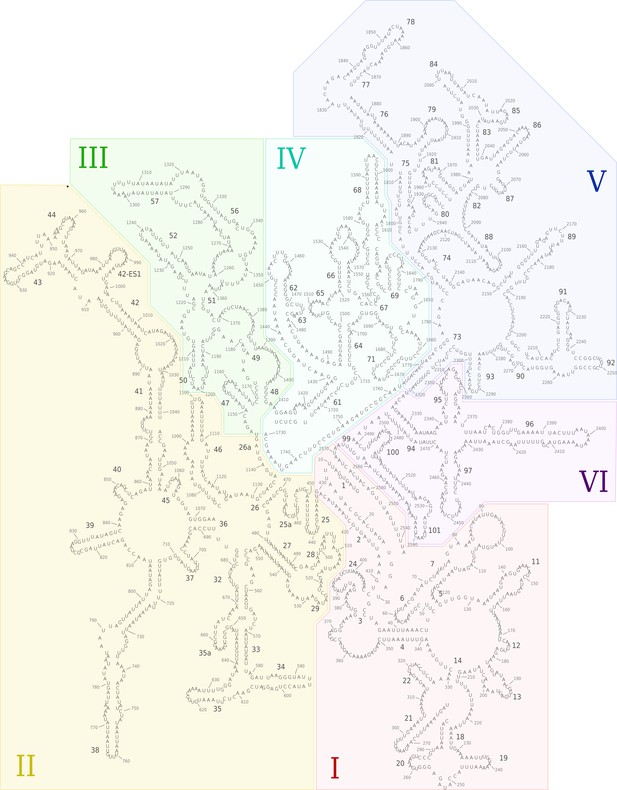
Secondary structure diagram of the T. thermophila LSU rRNA.
The rRNA is colored by domain, and expansion segments are indicated. Fragmentation is found in H18.
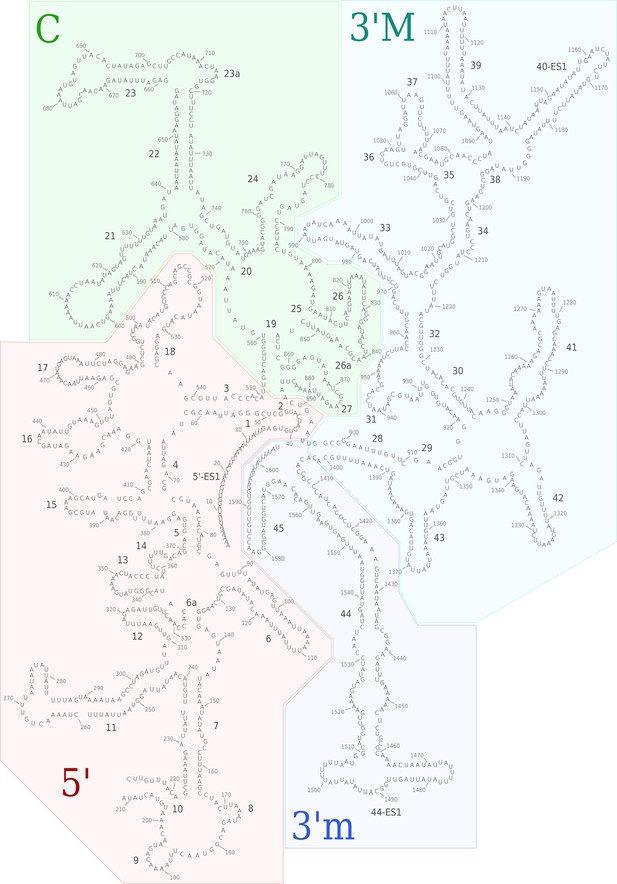
Secondary structure diagram of the T. thermophila SSU rRNA.
The rRNA is colored by domain, and expansion segments are indicated. Fragmentation is found in h10.
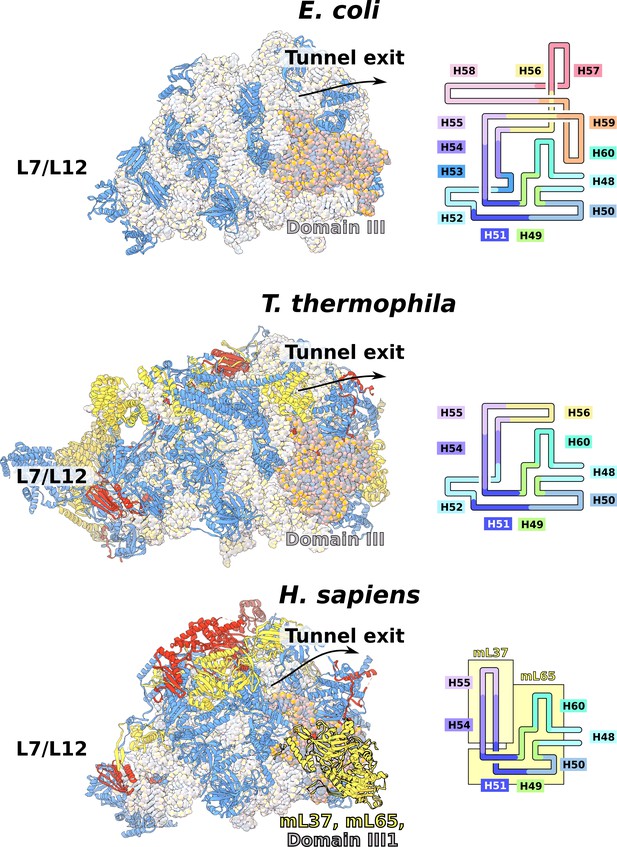
Evolution of rRNA domain III reduction.
Comparison of H48-60 rRNA between E. coli and mitochondria of T. thermophila and H. sapiens. Left, LSU model with rRNA is shown in gray and domain III is highlighted, conserved proteins are in blue, shared mitoribosomal proteins are in red, and specific mitoribosomal proteins are in yellow. Right, schematic representation of the rRNA region subjected to reduction. In H. sapiens, the rRNA deletions are structurally restabilized by proteins mL37 and mL65. In T. thermophila, the rRNA reduction is less severe, and flexible elements such as H56 have no binding protein partners, suggesting an intermediate stage between E. coli and H. sapiens.
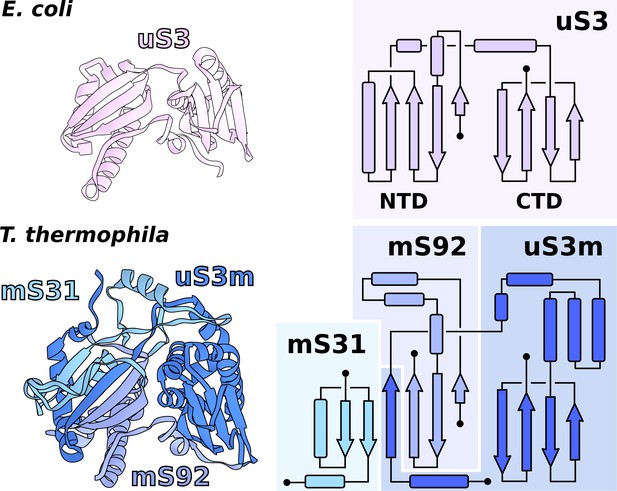
Functional uS3m consists of three separate proteins encoded in the nuclear and mitochondrial genomes.
Comparison of uS3 between E. coli ribosome and T. thermophila mitoribosome. The topology diagram of uS3 shows the organization of its domains that are replaced by three different proteins in T. thermophila. Mitoribosomal mS31 (nuclear encoded), mS92 (nuclear encoded), and one strand from uS3m (mitochondria encoded) collectively correspond to uS3-NTD, whereas mitoribosomal uS3m corresponds to uS3-CTD.
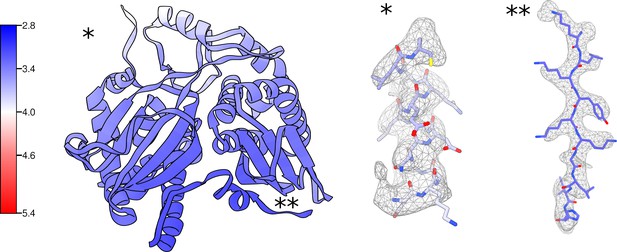
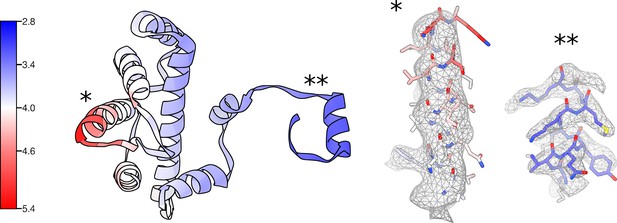
Local resolution and density for the uS3m module.
Proteins uS3m, mS31, and mS92 colored by local resolution with corresponding local density for regions with relatively low (*) and high (**) resolution.
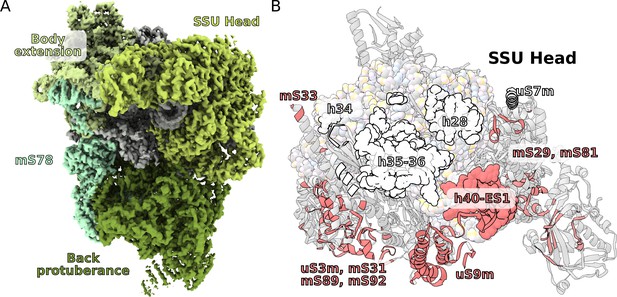
Unique functional features provide a distinct architecture of the mitoribosomal subunits.
(A) The overall structure of the SSU is substantially affected by the back protuberance and body extension that are bound to the head and interconnected through mS78. (B) The contact area between the SSU head and body is illustrated on the head. Mitochondria specific contacts are shown in red, and conserved contacts are shown in white.

SSU head interactions with body extension.
The SSU head and body extension form tight interactions through proteins uS9m, mS23, mS29, m31 and rRNA h40ES1. Subsequently, the two moieties are linked together.
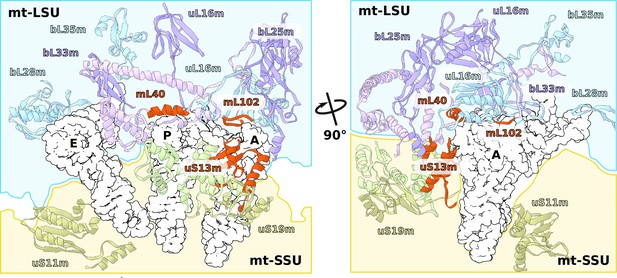
Specific protein elements interacting with tRNA binding sites.
The conventional tRNA binding sites are indicated in white based on the canonical L-shape of tRNAs (PDBID: 5MDZ). Related proteins of the LSU and SSU are shown in blue/purple and white, respectively. Mitochondria specific elements encasing the tRNA binding sites are shown in red.
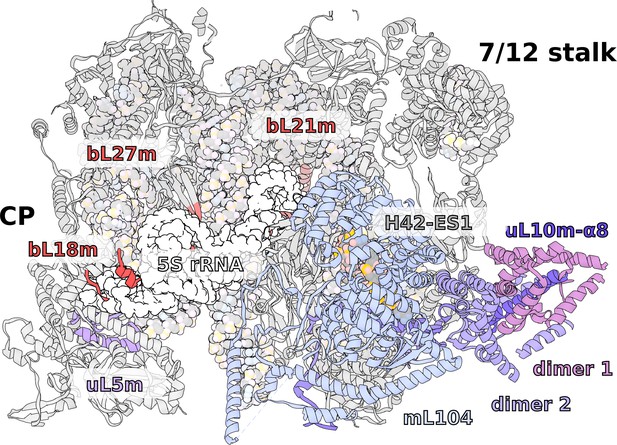
Specific features of the LSU.
The LSU central protuberance lacks 5S rRNA, and the L7/L12 stalk consists of only two dimers. Superposition of E. coli 5S rRNA reveals no substantial protein replacement, apart from minor elements shown in red. The model of the native L7/L12 stalk reveals unusual conformation due to the presence of rRNA expansion H42-ES1 and mL104.

Structure of the L7/L12 stalk.
(A) Helical repeat protein mL104 bound to H42-ES1 forms a stabilizing interface for the proximal bL12m dimer, which results in a more rigid structure. (B) Comparison of uL10 between E. coli ribosome and T. thermophila mitoribosome shows that the linker domain α8 is straight and rigid, lacking the representative kinks from bacteria.
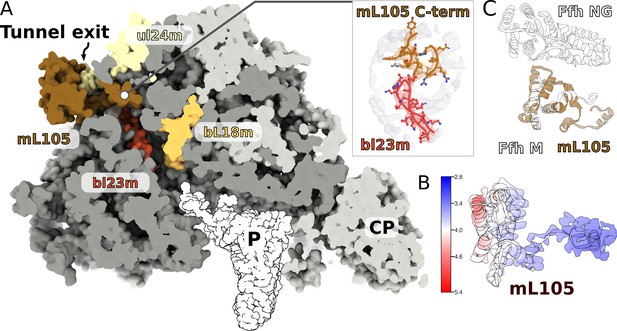
A signal peptide-binding domain protein is bound to the LSU.
(A) Slicing through the LSU shows the tunnel path and the targeting protein mL105 bound at the tunnel exit. The protein mL105 is positioned in a way that would affect the nascent polypeptide path. Inset displays interactions between mL105 CTD and bL23m close to the tunnel exit. (B) Model and density for mL105 colored by local resolution. (C) Superposition of the ribosome-bound Ffh M-domain (PDBID: 5GAF) with mL105 shows structural similarity.
Videos
Structure of the ciliate mitoribosome.
Additional files
-
Supplementary file 1
Cryo-EM data collection, refinement and validation statistics.
- https://cdn.elifesciences.org/articles/59264/elife-59264-supp1-v2.pdf
-
Supplementary file 2
Summary of the mitoribosomal proteins.
- https://cdn.elifesciences.org/articles/59264/elife-59264-supp2-v2.pdf
-
Transparent reporting form
- https://cdn.elifesciences.org/articles/59264/elife-59264-transrepform-v2.pdf