Early postmortem mapping of SARS-CoV-2 RNA in patients with COVID-19 and the correlation with tissue damage
Figures
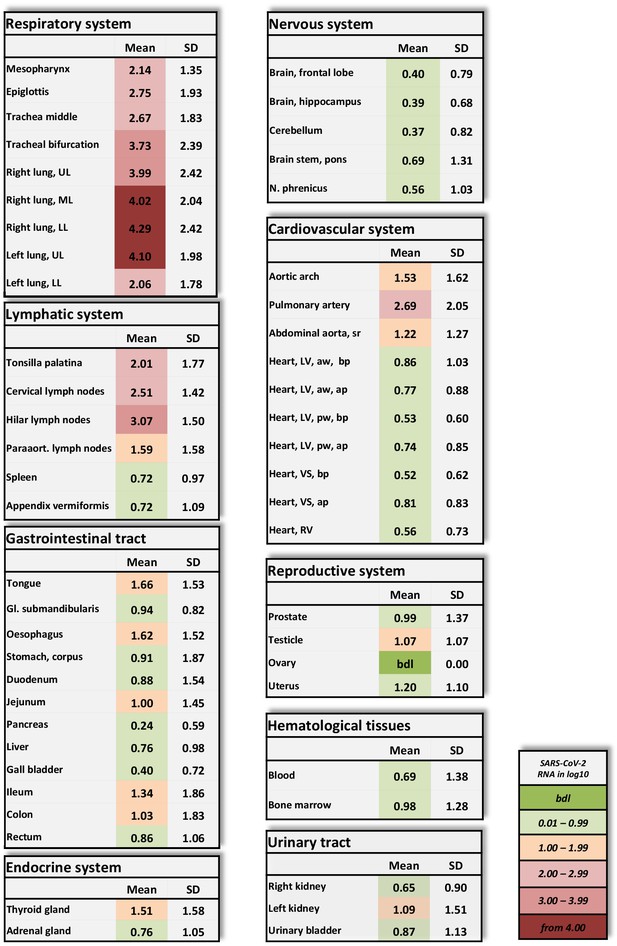
Overview of SARS-CoV-2 vRNA throughout the human body.
Postmortem determination of SARS-CoV-2 RNA with qRT-PCR of homogenized organs and tissues in copies/ml represented as decadic logarithm of 11 patients with mean value and standard deviation (SD) of the following systems: respiratory system, lymphatic system, gastrointestinal tract, urinary tract, nervous system, cardiovascular system, hematological tissues, reproductive system, and endocrine system. Intensity of colors describes the amount of vRNA. Abbrev.: bdl (below detection limit), UL (upper lobe), ML (middle lobe), LL (lower lobe), LV (left ventricle), sr (suprarenal), VS (ventricular septum), RV (right ventricle), aw (anterior wall), pw (posterior wall), bp (basal part), ap (apical part), paraaort. (paraaortal).
-
Figure 1—source data 1
Postmortem determination of SARS-CoV-2.
RNA with qRT-PCR of homogenized organs and tissues with r-Biopharm qRT-PCR, raw data shows the crossing points (cycle threshold, Ct).
- https://cdn.elifesciences.org/articles/60361/elife-60361-fig1-data1-v1.xlsx
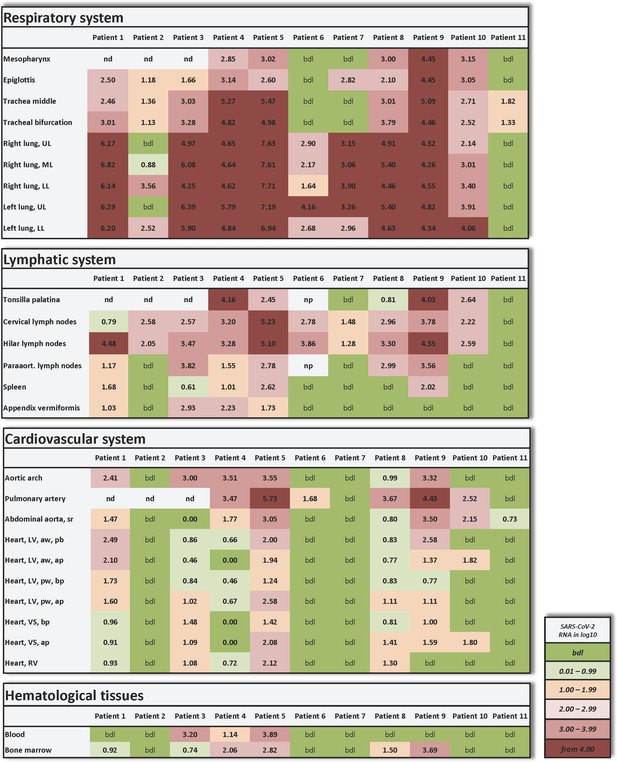
Individual vRNA load of the respiratory system, the lymphatic system, the cardiovascular system, and hematological tissues.
Postmortem determination of SARS-CoV-2 RNA with qRT-PCR of homogenized organs and tissues in copies/ml represented as decadic logarithm of 11 patients. Abbrev.: bdl (below detection limit), UL (upper lobe), ML (middle lobe), LL (lower lobe), LV (left ventricle), sr (suprarenal), VS (ventricular septum), RV (right ventricle), aw (anterior wall), pw (posterior wall), bp (basal part), ap (apical part), nd (not determined), np (not present). Intensity of colors describes the amount of RNA.
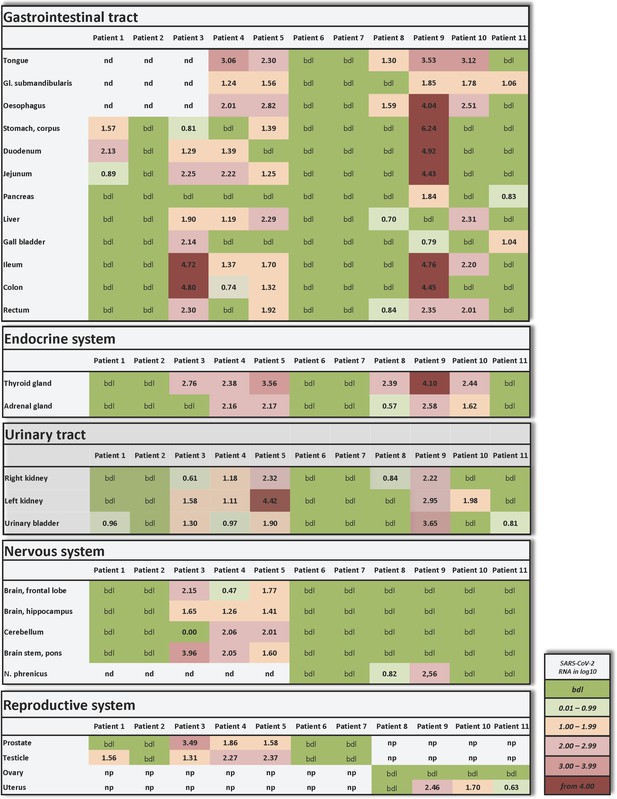
Individual vRNA load of the gastrointestinal tract, the endocrine system, the urinary tract, the nervous system, and the reproductive system.
Postmortem determination of SARS-CoV-2 RNA with qRT-PCR of homogenized organs and tissues in copies/ml represented as decadic logarithm from patient 1–11. Abbrev.: bdl (below detection limit), nd (not determined), np (not present). Intensity of colors describes the amount of vRNA.
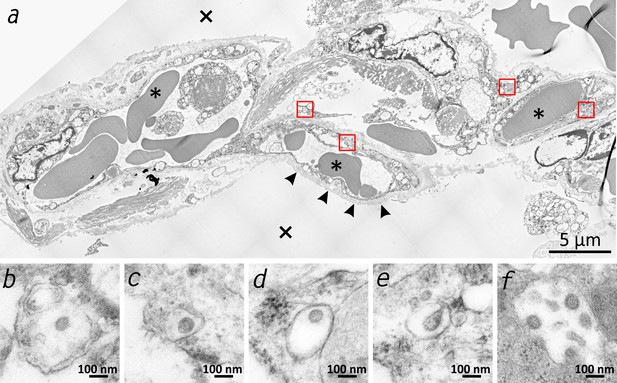
Transmission electron microscopic image of the lung tissue of patient 3.
(a) Alveolar septum showing intact capillaries with erythrocytes (asterisk) and the air space (cross). The blood-air barrier is damaged as the pneumocytes are missing and the basal membrane is exposed to air (arrowheads). (b–e) Close-ups of the four boxed regions in (a) from left to right showing SARS-CoV-2 virus particles encased in plasmatic vesicles of alveolar fibrocytes. (f) Reference image of SARS-CoV-2 virus particles proliferated in cell culture (Vero76).
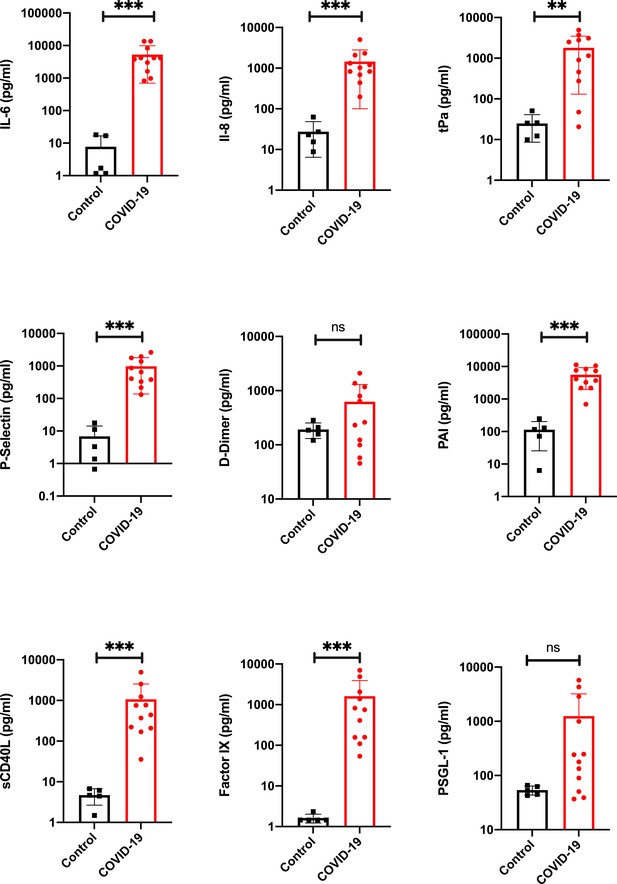
Proinflammatory and prothrombotic factors.
Blood analysis of patients 1–11 by using Legendplex Panel (Biolegend, CA, USA) of the proinflammatory cytokines Interleukin (IL)−6 (a) and IL-8 (b) as well as tissue plasminogen activator (tPa) (c), P-Selectin (d), D-Dimer (e), Plasminogen activator inhibitor-1 (PAI) (f), soluble (s) CD40ligand(L) (g), Factor IX (h), and the P-selectin glycoprotein ligand 1 (PSGL-1) (i) in pg/ml compared to the mean of five controls (Control, healthy volunteers). Unpaired t-test, Mann-Whitney test p<0.005 ***; p<0.05 **; ns=not significant.
-
Figure 3—source data 1
Blood analysis of patients 1–11 by using the Legendplex Panel (BioLegend, san Diego, CA, USA) .
Panel (Biolegend, CA, USA) of the proinflammatory cytokines Interleukin (IL)−6 (a) and IL-8 (b) as well as tissue plasminogen activator (tPa) (c), P-Selectin (d), D-Dimer (e), Plasminogen activator inhibitor-1 (PAI) (f), soluble (s) CD40ligand(L) (g), Factor IX (h) and the P-selectin glycoprotein ligand 1 (PSGL-1) (i) in pg/ml compared to the mean of five controls (Control, healthy volunteers). Raw data of the Legendplex software with standard curves.
- https://cdn.elifesciences.org/articles/60361/elife-60361-fig3-data1-v1.xls
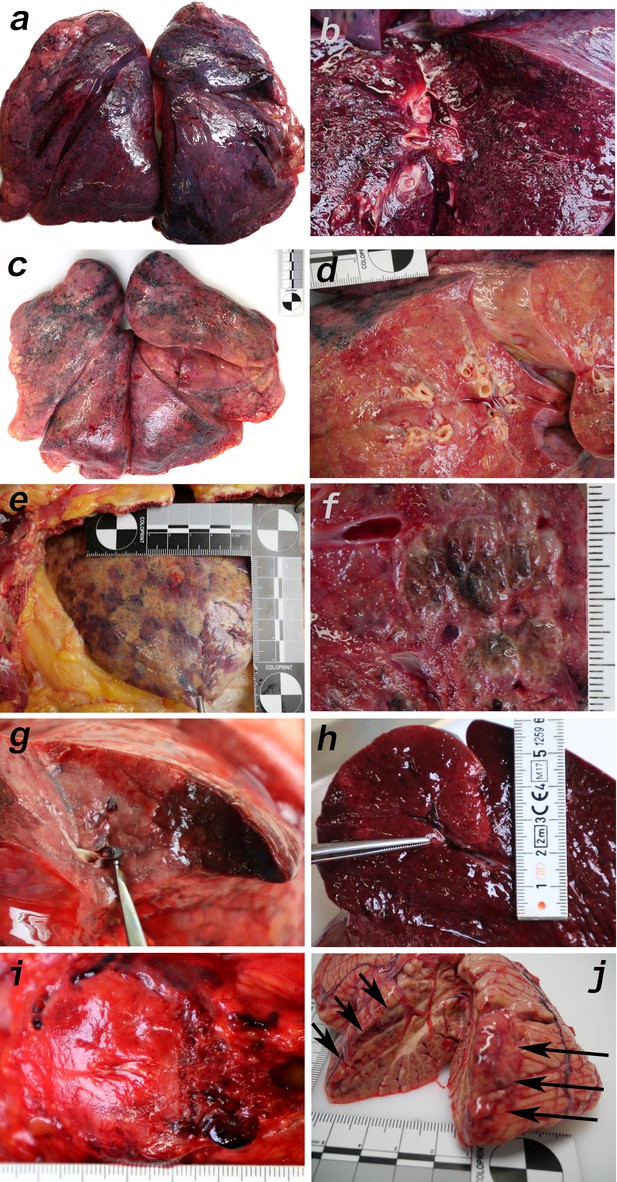
Macromorphology findings of COVID-19 patients.
(a) Pneumonectomy of patient one showed strong congestion with liquids and hemorrhages. The tissue consistency was fragile. (b) Cut surface of lung tissue in higher magnification as shown in (a). The pleura shows further hemorrhages. (c) Pneumonectomy of patient seven showed a more solid lung tissue without congestion. The tissue consistency was very firm. (d) Cut surface of lung tissue in higher magnification as shown in (c). Lung tissue was retracted adjacent to the bronchus. (e) Pale pleura visceralis of the lung of patient 6 with disseminated hemorrhages and signs of disturbed ventilation. (f) Nodular transformation of lung tissue as phenomenon of fungal superinfection in patient 3. (g) Hemorrhagic lung infarct in patient 4 due to a thrombembolus in a pulmonary artery branch. (h) Anemic spleen infarct due to a clotted small artery in patient 4. (i) Fulminant stasis and thromboses in the periprostatic plexus in patient 4. (j) Cerebellar infarction (hemorrhagic) in patient 9.
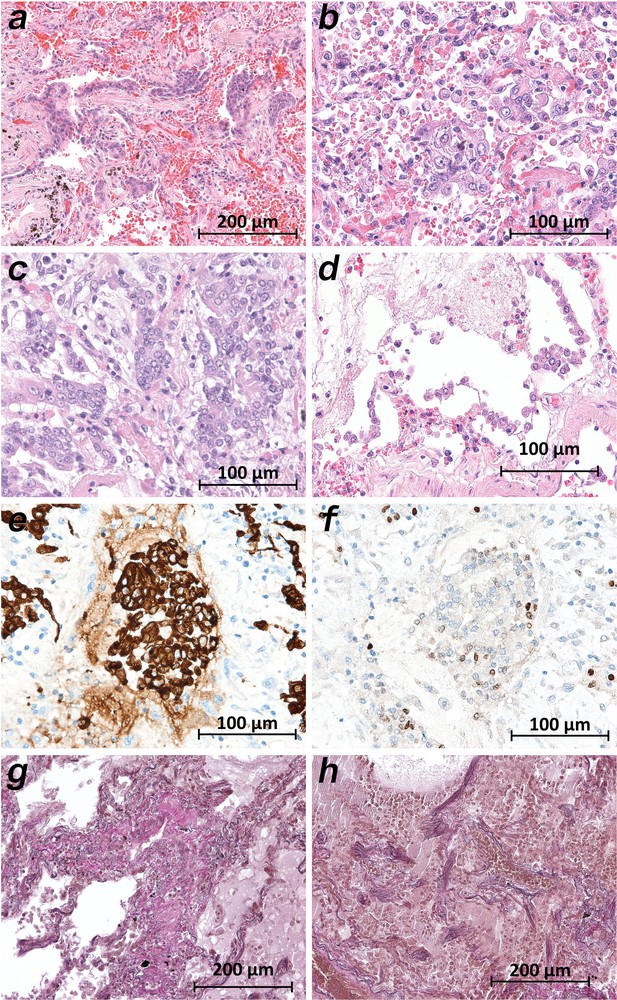
Micromorphology lung findings of COVID-19 patients.
(a) Destroyed lung tissue with intraalveolar hemorrhagia and aggregates of prominent epithelial cells resembling squamous metaplasia (patient 1; HE). (b) Strong architectural damage of lung alveolar tissue with disruption of the epithelial barrier and intraalveolar accumulation of enlarged cells with prominent nuclei and visible nucleoli. Initial syncytial pattern is given (patient 2; HE). (c) Lung tissues with multinucleated giant cells admixed with only few lymphocytes (patient 4; HE). (d) Alveolar unit with band-like desquamation of the alveolar epithelial cells in the alveolar space partially filled with liquids, erythrocytes and few lymphocytes (patient 5; HE). (e) Multinucleated giant cell in an alveolar space is strongly positive for keratins (patient 4; immunostaining AE1/3). (f) Serial section of (e), the multinucleated giant cell after immunostaining against TTF1 (patient 4; immunostaining TTF1). (g) Lung tissue with interstitial fibrosis (patient 8; EvG). (h) Lung tissue with interstitial and intraalveolar fibrosis (patient 8; EvG).
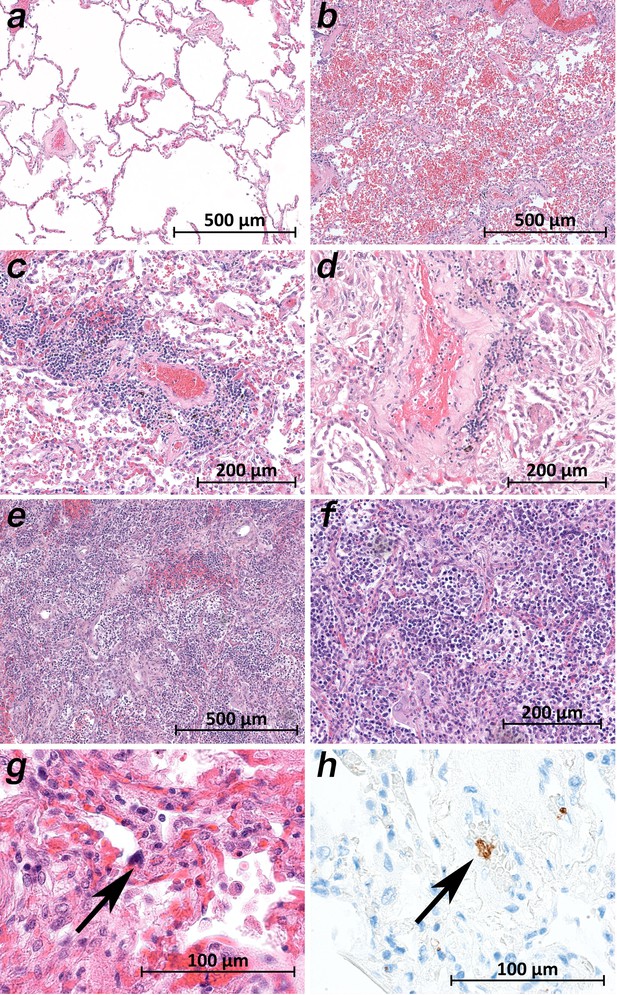
Micromorphology lung findings of COVID-19 patients.
(a) Lung tissue with minimal emphysematous changes derived from the upper lobes without detectable viral loads (patient 2; HE). (b) Severe hemorrhagic pneumonia in specimens from the lower lobes with high viral loads (patient 2; HE). (c) Vasculitis-like changes around pulmonary artery branches (patient 4; HE). (d) Damaged lung tissue with hemostasis and inflammatory changes adjacent to the pulmonary artery branch (patient 4; HE). (e) Strong lymphocytic-predominant infiltration of lung tissues with hemorrhagic and interstitial edema (patient 7; HE). (f) Higher magnification of the lymphocytic-predominant infiltrate (patient 7; HE). (g) Lung tissue with a large nucleated cell in an alveolar capillary, suggestive for a megakaryocyte (arrow; patient 9; HE). (h) The same tissue after immunostaining against CD61 (patient 9; immunostaining CD61).
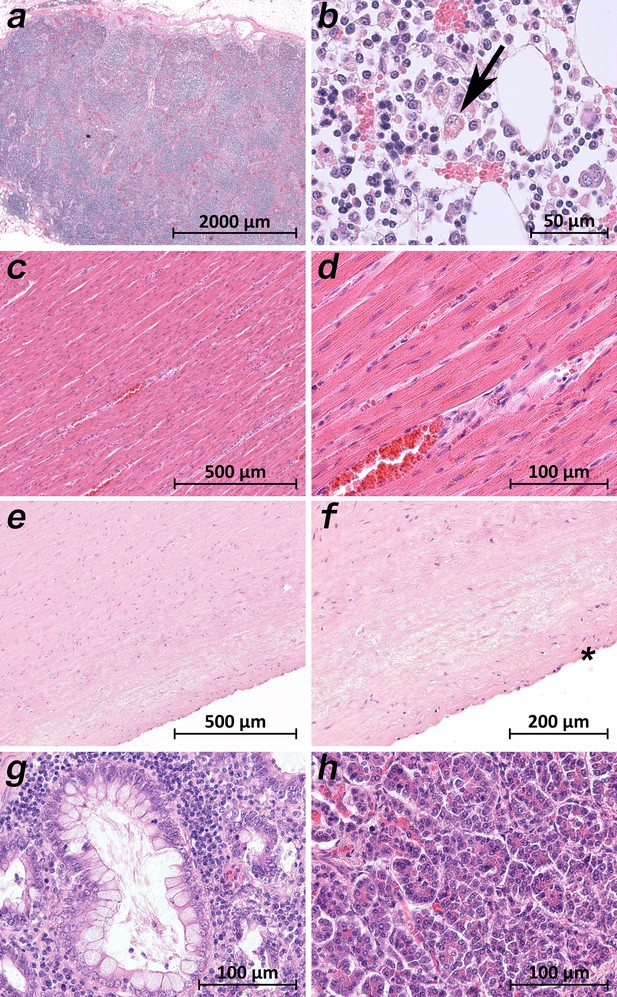
Extrapulmonary micromorphology findings of COVID-19 patients.
(a) Overview of a mediastinal lymphnode with some nodular aggregates of lymphocytes, but destroyed lymphofollicular structures (patient 5; HE). (b) Bone marrow with prominent hemophagocytosis (arrow) and maturating cells of the hematopoiesis (patient 9; HE). (c) Myocardial tissue of the left ventricle (patient 1; HE). (d) Myocardial tissue of the left ventricle in higher magnification with a minimal increase in cellularity indicating for an activated cardiomesenchyme (patient 1; HE). (e) Thoracic aorta with a low number of non-inflammatory nucleated cells in an unsuspicious matrix (patient 5; HE). (f) Tissue from the thoracic aorta in higher magnification. The endothelium is labeled with an asterisk (patient 5; HE). (g) Colon mucosa with a crypt lined by goblet cells and enterocytes without any strong intraepithelial inflammation (patient 3; HE). (h) Exocrine pancreas tissue with structural intact acini without inflammatory cells (patient 9; HE).
Tables
Clinical characteristics of deceased COVID-19 patients.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Sex | m | m | m | m | m | m | m | f | f | f | f |
| Age [years] | 82 | 66 | 78 | 54 | 80 | 64 | 64 | 87 | 83 | 85 | 52 |
| BMI [kg/m²] | 23.8 | 31.5 | 25.2 | 28.3 | 28.8 | 35.4 | 24.6 | 24.6 | 28.9 | 26.2 | 21.4 |
| Pre-existing medical conditions | AF, DM,autoimmune pancreatitis,purpura pigmentosa | aHT | aHT, DM, CRF, PAD, urosepsis shortly before COVID-19 | None known | DM, CRF, CHF | GPA, CRF, COPD, aHT, AF, DM | AS, COPD | CHF, CRF, DM, past stroke epilepsy,erysipelasshortly before COVID-19 | aHT, DM, CRF, 1-vessel-CHD, AF, PAD | CHF, CRF, DM | Cervical carcinoma |
| Hospitalization [d] | 7 | 5 | 30 | 10 | 9 | 20 | 15 | 12 | 7 | 9 | 4 |
| ICU [d] | 7 | 5 | 7 | 8 | 8 | 17 | 9 | 0 | 0 | 0 | 4 |
| Mechanical ventilation [d] | 7 | 4 | 7 | 7 | 8 | 16 | 9 (ECMO) | 0 | 0 | 0 | 0 |
| Antiviral drugs | Lopinavir, ritonavir | Lopinavir, ritonavir | None | None | None | Lopinavir, ritonavir | Lopinavir, ritonavir | None | None | None | None |
| Cause of death (acc. to clinic) | MOF | Pulmonary embolism | MOF | Lung failure | Suspected myocardial infarction | Lung failure | Lung failure | Respiratory failure | Pneumonia | Pneumonia | Ileus |
-
aHT – arterial hypertension, AF – atrial fibrillation, AS – atherosclerosis, CHF – chronic heart failure, CHD – coronary heart disease, COPD – chronic obstructive pulmonary disease, CRF – chronic renal failure, DM – diabetes mellitus, ECMO - Extracorporeal membrane oxygenation, f – female, GPA – granulomatosis with polyangiitis (Wegener’s Granulomatosis), ICU – intensive care unit, MOF – multiple organ failure, PAD – peripheral artery disease.
Autopsy findings.
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | Patient 7 | Patient 8 | Patient 9 | Patient 10 | Patient 11 | ||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMI [h] | 3.5 | 2.25 | 7.5 | 9.5 | 15.0 | 2.33 | 1.5 | 5.8 | 5.0 | 6.0 | 3.5 | |
| Lung weight | R 1550 g L 1240 g | R 940 g L 760 g | R 1170 g L 790 g | R 1860 g L 1640 g | R 1370 g L 990 g | R 970 g L 570 g | R 890 g L 770 g | R 610 g L 480 g | R 550 g L 680 g | R 530 g L 400 g | R 760 g L 590 g | |
| Lung macro* | Edema | +++ | ++ | ++ | ++ | ++ | + | + | + | + | ++ | + |
| Hyperemia | +++ | + | +++ | ++ | ++ | + | + | + | + | ++ | + | |
| Hemorrhage | +++ | + | +++ | ++ | ++ | ++ | + | - | + | ++ | + | |
| Texture | reduced | reduced | reduced | reduced | reduced | enhanced | enhanced | enhanced | reduced | reduced | reduced | |
| Infarction | - | - | + | + | - | - | - | - | - | - | - | |
| Lung micro* | DAD, exsudative phase | +++ | ++ | ++ | ++ | +++ | + | + | + | + | ++ | + |
| DAD, proliferative phase | ++ | - | - | + | + | +++ | +++ | +++ | ++ | ++ | - | |
| Multinucleated giant cells | +++ | ++ | ++ | ++ | ++ | + | + | - | ++ | + | + | |
| Squamous metaplasia | +++ | + | ++ | ++ | ++ | + | ++ | ++ | ++ | +++ | + | |
| Megakaryocytes | - | - | - | - | - | + | + | ++ | + | - | + | |
| Lymphocytic infiltrates | + | + | - | - | ++ | - | +++ | - | - | + | - | |
| Vasculitis | - | + | + | + | - | - | - | + | + | - | - | |
| Stasis / fibrin thrombi | + | + | - | - | - | - | - | + | + | + | tumor | |
| Emboli | - | ++ | - | + | + | - | - | - | - | + | tumor | |
| Superinfection | - | - | Fungal | - | - | bacterial | - | - | bacterial | bacterial | - | |
| Heart weight | 480 g | 600 g | 550 g | 430 g | 810 g | 610 g | 480 g | 380 g | 360 g | 300 g | 350 g | |
| Heart macro | Pericarditis, 2-v-CHD | conc. HT | 3-v-CHD | Unremarkable | exc. HT | exc. HT | - | 2-v-CHD | 3-v-CHD | 1-v-CHD | un- remarkable | |
| Geart micro | Moderate fibrosis | Moderate fibrosis | Chronic ischemia | Unremarkable | amyloidosis | slight fibrosis | slight fibrosis | slight fibrosis | moderate fibrosis | atrophy, fibrosis | invasive metastases | |
| Further autopsy findings (besides age-related or pre-existing) | Steatosis hepatis | NASH | - | CLL, ICH, spleen infarction | severe cardiac amyloidosis | hepatic siderosis | - | adrenal thrombosis | - | endometrial carcinoma (apT1a) | cervical carcinoma (apT4) | |
| Cause of death (acc. to autopsy)† | COVID-19 (HP) | COVID-19 (pulmonary embolism) | COVID-19 (HP) | COVID-19 (HP) | COVID-19 (HP) | COVID-19 (CCP) | COVID-19 (CCP) | COVID-19 (CCP) | COVID-19 (BP+HP) | COVID-19 (BP+HP) | malignant tumor disease | |
-
BP – bronchopneumonia, CCP – chronic carnifying pneumonia, CHD – coronary heart disease, CLL – chronic lymphatic leukemia, DAD – diffuse alveolar damage, HP – hemorrhagic pneumonia, HT – hypertrophy, conc. = concentric, exc. = excentric, ICH – intracerebral hemorrhage, NASH – non-alcoholic steatosis hepatitis, PMI – postmortem interval (time between death and autopsy), v – vessel.
*Semi-quantitative evaluation: no (-), few (+), moderate (++), very much (+++).
-
†Supplemented term within brackets describes the dominant finding that caused death by COVID-19.





