Charge-driven condensation of RNA and proteins suggests broad role of phase separation in cytoplasmic environments
Abstract
Phase separation processes are increasingly being recognized as important organizing mechanisms of biological macromolecules in cellular environments. Well-established drivers of phase separation are multi-valency and intrinsic disorder. Here, we show that globular macromolecules may condense simply based on electrostatic complementarity. More specifically, phase separation of mixtures between RNA and positively charged proteins is described from a combination of multiscale computer simulations with microscopy and spectroscopy experiments. Phase diagrams were mapped out as a function of molecular concentrations in experiment and as a function of molecular size and temperature via simulations. The resulting condensates were found to retain at least some degree of internal dynamics varying as a function of the molecular composition. The results suggest a more general principle for phase separation that is based primarily on electrostatic complementarity without invoking polymer properties as in most previous studies. Simulation results furthermore suggest that such phase separation may occur widely in heterogenous cellular environment between nucleic acid and protein components.
Introduction
Biological cells compartmentalize to support specific functions such as stress response (Boulon et al., 2010; Protter and Parker, 2016), regulation of gene expression (Boisvert et al., 2007; Morimoto and Boerkoel, 2013), and signal transduction (Su et al., 2016). Compartmentalization by organelles that are surrounded by lipid membranes is well known. In addition, membrane-less organelles that result from coacervation have been described (Alberti et al., 2019; Banani et al., 2017; Boeynaems et al., 2018; Ditlev et al., 2018). In the nucleus, they include the nucleolus (Feric et al., 2016; Iarovaia et al., 2019), nuclear speckles (Galganski et al., 2017; Lamond and Spector, 2003), and cajal bodies (Cioce and Lamond, 2005; Gall, 2000; Machyna et al., 2013); stress granules (Protter and Parker, 2016; Burke et al., 2015; Molliex et al., 2015); germ granules (Brangwynne et al., 2009; Voronina et al., 2011); and processing bodies Fromm et al., 2014; Luo et al., 2018 have been found in the cytoplasm. The formation of coacervates via condensation and phase separation depends on the composition and concentration of the involved macromolecules (Ditlev et al., 2018) as well as environmental conditions such as pH, temperature, and the concentration of ions (Alberti et al., 2019; Elbaum-Garfinkle et al., 2015; Ruff et al., 2018). Multivalent interactions, the presence of conformationally flexible molecules (Sawyer et al., 2019; Radhakrishna et al., 2017; de Kruif et al., 2004), and electrostatic interactions between highly charged molecules (Sawyer et al., 2019; de Kruif et al., 2004; Fay and Anderson, 2018; Cummings and Obermeyer, 2018; Michaeli et al., 1957; Mattison et al., 1995) are well-known as the key factors that promote phase separation (PS), in particular via complex coacervation (Sing, 2017; Andreev et al., 2018). In biological environments, nucleic acids such as RNA have been found to play a prominent role in condensate formation due to their charge (Chujo et al., 2016; Clemson et al., 2009; Falahati et al., 2016; Mitrea et al., 2016; Smith et al., 2016; Van Treeck et al., 2018; Garcia-Jove Navarro et al., 2019). Another component often found in biological condensates are intrinsically disordered peptides (IDPs) that may phase separate alone or in combination with RNA (Ruff et al., 2018; Smith et al., 2016; Brady et al., 2017; Dignon et al., 2018a; Posey et al., 2018), although disorder may not be essential for phase separation (Sanders et al., 2020; Aumiller et al., 2016). Condensates often materialize as droplets, where experiments such as fluorescence recovery after photobleaching (FRAP) (Shin et al., 2017; Taylor et al., 2019) or direct visualization of merging droplets (Van Treeck et al., 2018; Li et al., 2012) may confirm liquid-like behavior. However, a variety of other types of less-liquid condensates involving biomolecules have been described including clusters, gels, and aggregation to fibrils or tangles (Molliex et al., 2015; Alberti and Hyman, 2016; Weber, 2017; Weber and Brangwynne, 2012; Lin et al., 2015; Jain et al., 2016). In those cases, internal diffusional dynamics may be highly retarded or lost. The high degree of polydispersity in biological multicomponent systems presents additional changes. An especially intriguing aspect of polydisperse systems is the propensity for multiphasic behavior (Feric et al., 2016; Sanders et al., 2020; Lu and Spruijt, 2020), which imparts a potential for fine-grained tunable spatial patterning of biomolecules in cellular systems (Sanders et al., 2020).
Biomolecular condensates have been studied extensively (Mitrea et al., 2018a). Microscopy (Elbaum-Garfinkle et al., 2015; Banani et al., 2016; Nott et al., 2015), nuclear magnetic resonance (NMR) spectroscopy (Burke et al., 2015; Brady et al., 2017), fluorescence spectroscopy (Feric et al., 2016; Mitrea et al., 2018b; Wei et al., 2017), X-ray diffraction (Kato et al., 2012; Lin et al., 2016a), and scattering methods Mitrea et al., 2018b; Li et al., 2012; Riback et al., 2017 have characterized in vitro (Feric et al., 2016; Burke et al., 2015; Elbaum-Garfinkle et al., 2015; Brady et al., 2017; Nott et al., 2015; Wei et al., 2017; Kato et al., 2012; Lin et al., 2016a; Riback et al., 2017) and in vivo systems (Brangwynne et al., 2009; Brangwynne et al., 2011; Maharana et al., 2018). Theoretical studies have complemented experiments (Mitrea et al., 2018a; Dignon et al., 2019), including particle-based simulations (Dignon et al., 2018b) and analytical approaches based on polymer (Posey et al., 2018; Brangwynne et al., 2015) and colloid theories (Nguemaha and Zhou, 2018; Qin and Zhou, 2017; Woldeyes et al., 2017). Additional insights into specific interactions have come from molecular dynamics (MD) simulation studies (Wei et al., 2017; Rauscher and Pomès, 2017; Pak et al., 2016). Polymer aspects of IDPs and unstructured RNA were emphasized in applications of Flory-Huggins theory in combination with simulations (Feric et al., 2016; Dignon et al., 2018b; Fei et al., 2017; Lin et al., 2016b). Related studies in the colloid field have described the phase behavior of macromolecules and nanoparticles as single spherical particles (Nguemaha and Zhou, 2018; Qin and Zhou, 2017; Woldeyes et al., 2017). However, most of the latter studies so far have focused on liquid-solid transitions and the formation of finite size clusters in monodisperse systems. Despite progress, it has remained unclear what components can lead to condensation, especially in highly heterogeneous cellular environments.
As most previous studies have focused on specific biomolecules undergoing PS, we focus here on the question of how general of a phenomenon PS may be in biological environments and what factors may determine the propensity for PS in a heterogeneous system. The starting point is a molecular model of a bacterial cytoplasm that was established by us previously (Feig et al., 2015; Yu et al., 2016) and that was simulated here again but using colloid-like spherical particles with a potential parameterized against atomistic MD simulations of concentrated protein solutions. Coarse-grained modeling of cytoplasmic environments has a long history of impressive earlier efforts (Ridgway et al., 2008; McGuffee and Elcock, 2010; Ando and Skolnick, 2010; Wang and Cheung, 2012; Xu et al., 2013; Hasnain et al., 2014; Trovato and Tozzini, 2014; Bicout and Field, 1996) as reviewed in more detail elsewhere (Feig et al., 2017; Feig and Sugita, 2019). However, the time and spatial scales covered here are more extensive than in previous work, allowing us to focus on PS processes. We found that distinct phases enriched with highly negatively charged RNA and positively charged proteins were formed in the simulations, consistent with a generic electrostatic mechanism that does not require specific interaction sites or elements of disorder and may apply broadly to mixtures of nucleic acids and proteins. The phase behavior seen in the cytoplasmic system was reproduced in reduced five- and two-component models and described by an analytical model where we could systematically vary molecular charge, size, and concentrations. The main prediction of the formation of condensates between RNA and positively charged proteins was confirmed experimentally via confocal microscopy and FRET spectroscopy and the nature of the condensates was analyzed further via dynamic light scattering and nuclear magnetic resonance spectroscopy. The details of the findings from simulation, theory, and experiment are described in the following.
Results
Condensates enriched in tRNA and ribosomes form in a model bacterial cytoplasm
A model of the cytoplasm of Mycoplasma genitalium established previously (Feig et al., 2015; Yu et al., 2016) was simulated at a coarse-grained (CG) level with one sphere per macromolecule or complex (Supplementary file 1). CG particle interactions were calibrated against results from atomistic MD simulations of concentrated protein solutions. The parameters involve only two particle-dependent properties, namely size and charge. Droplet-like condensates formed spontaneously within 20 µs (Figure 1A/B) and remained present during 1 ms simulation time. Similar results were obtained with an alternate effective charge model that resulted in better agreement between theory and experiment (see below; Figure 1—figure supplement 1) Two types of condensates were observed: one type contained predominantly tRNAs and positively charged proteins; the other type contained ribosome particles (RP) and positively charged proteins. The RP condensates also attracted the weakly negatively charged GroEL particles at the surface (Figure 1A). The condensates increased in size as the system size was increased from 100 to 300 nm (Figure 1A). This observation is consistent with PS rather than finite-size cluster formation. The presence of multiple droplets in the 300 nm system suggests incomplete convergence, but as the droplets grow in size, further merging becomes kinetically limited due to slowing diffusion. We did not find evidence for growth via Ostwald ripening where particles preferentially evaporate from smaller condensates and redeposit onto larger condensates. Further analysis focused on the condensates observed in the 100 nm system.
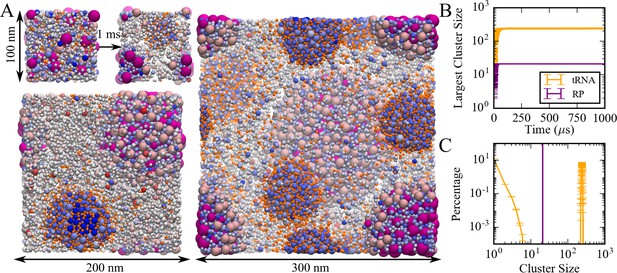
Coarse-grained simulations of a model bacterial cytoplasm.
(A) Initial and final frames for 100 nm box and final frames for 200 and 300 nm boxes are shown with tRNAs in orange, ribosomes in magenta, and other molecules colored according to their charges (blue toward positive charges; red toward negative charges). Sphere sizes are shown proportional to molecular sizes. Large pink spheres correspond to GroEL particles. (B) Size of the largest cluster vs. simulation time in 100 nm system. (C) Cluster size distributions for tRNA and RP during the last 500 µs in the 100 nm system.
Cluster analysis considered interactions between the nucleic acids and positively charged proteins to obtain trajectory-averaged cluster size distributions (Figure 1C). Most tRNA (87%) was part of a condensate. The remaining fraction of tRNA existed as monomers or small clusters, suggesting coexistence of dilute and condensed phases. RP were only found in the RP condensates. Total macromolecular volume fractions inside tRNA and RP condensates were 0.42 and 0.58, respectively, whereas volume fractions for just tRNA and RP inside their respective condensates were 0.07 and 0.26. The volume of the condensates was estimated based on the overlapping van der Waals volumes of spheres inside the largest cluster with an additional probe of 2.2 nm in consistent with our cluster definition. The dilute phase volume was estimated as the remaining accessible volume after subtracting volume of condensates from the total volume. The condensates had significantly higher macromolecular densities than the rest of the simulated system (Figure 1—figure supplement 2). The moderately high volume fractions for tRNA condensates are still within the range of concentrated liquid phases (Dumetz et al., 2008), but the higher volume fractions in the RP condensate tend toward solid- or gel-like phases (Dumetz et al., 2008). Radial distribution functions of tRNA and RP from the center of the corresponding condensates show a relatively smooth decay with a soft boundary for tRNA condensates (Figure 1—figure supplement 3), that are consistent with a more dynamic phase, whereas distinct peaks and a sharper boundary for RP indicate a highly ordered arrangement in the RP condensates. The more ordered structure of the RP condensates may be an example of the kind of structured condensates resulting from a balance between homotypic and heterotypic interactions as described recently (Regy et al., 2020).
We observed separate condensates involving tRNA or RP, presumably due to the large difference in size of RP vs. tRNA that may be explained at least in part by the Asakura-Oosawa depletion model (Asakura and Oosawa, 1958). Both tRNA and RP condensates contained (positively charged) proteins at high concentrations. tRNA and RP interactions with those proteins were favorable as evidenced by a strong peak in the pairwise radial distribution function g(r) at contact distance (Figure 1—figure supplement 4). The charge and size of the proteins attracted to the condensates differed between tRNA and RP condensates (Figure 1—figure supplement 5). In the tRNA condensates, large proteins with radii of 3 nm and above and with charges of 10 and above were preferred. In contrast, the proteins in the RP condensates were smaller, with radii of 3 nm or less, and many proteins had charges below 10. This suggests that differential interactions between different size and charge nucleic acid and protein particles may further explain the formation of separate condensates involving tRNA and RP.
The dynamics inside and outside the condensates was analyzed in terms of translational diffusion coefficients (Dtr) calculated based on mean-squared displacements (Figure 1—figure supplement 6). Diffusion during the last 1 μs of the simulation was compared with diffusion during the first 1 μs when condensates were not yet formed. Molecule-specific values of Dtr are given in Supplementary file 1. As a function of the radius of the macromolecules (Figure 1—figure supplement 7), Dtr values follow a similar trend as observed before in atomistic simulations of the same system. Diffusion outside the condensates resembled diffusion in the dispersed phase. In tRNA condensates, the diffusion of macromolecules is similar to the dispersed phase or is moderately retarded, depending on the molecule, and consistent with reduced diffusion in increased protein concentrations seen in experiment (Muramatsu and Minton, 1988; Zimmerman and Minton, 1993). In RP condensates, diffusion is reduced to a greater extent, but significant dynamics is still maintained for most types of macromolecules as they diffuse around a relatively static RP cluster (Video 1).
Simulation of bacterial cytoplasm model.
Trajectory of the 100 nm system during the last 1 µs of a 1 ms simulation with tRNAs in orange, ribosomes in magenta, and other molecules colored according to their charges (blue toward positive charges; red toward negative charges). Sphere sizes are shown proportional to molecular sizes. Large pink spheres correspond to GroEL particles.
Factors promoting RNA condensation in a reduced five-component model system
A simplified five-component system was constructed to reproduce the RNA condensation observed in the cytoplasmic model. The simplified model consisted of tRNA, ribosome particles (RP), large (POSL, q = 20, r = 3.5 nm) and small (POSS, q = 1, r = 2.52 nm) positively charged proteins as well as neutral crowders (CRW, q = 0, r = 2.52 nm). tRNA and RP concentrations were initially set as in the cytoplasmic model while concentrations, sizes, and charges of the other three particle types were adjusted to match the total number of particles, total molecular volume, and total charge of the cytoplasmic system as closely as possible. Subsequently, a series of simulations were run at different concentrations and with different parameters (Supplementary file 2).
In simulations of the five-component model, tRNA and RP condensed separately as in the cytoplasmic model (Figure 2—figure supplement 1). Again, the condensates formed quickly, within 50 μs (Figure 2—figure supplement 1), and cluster size distributions of tRNA and RP resembled the results from the cytoplasmic system (cf. Figure 1 and Figure 2—figure supplement 1). However, in contrast to the cytoplasmic system, we found a small fraction (2% on average) of RP in the dilute phase. As in the cytoplasmic model, tRNA strongly preferred interactions with the larger POSL particles, whereas RP interacted favorably with both POSS and POSL (Figure 2—figure supplement 2). tRNA condensates remained highly dynamic as in the cytoplasmic system. From the last 100 μs of the simulation, we obtained diffusion coefficients Dtr for tRNA of 28.3 ± 0.7 and 59.0 ± 0.5 nm2/µs inside and outside of the condensates, respectively, similar to values of 16.3 ± 0.1 and 55.5 ± 0.8 nm2/µs in the cytoplasmic system. Diffusion coefficients for RP inside and outside of the RP condensates were 0.49 ± 0.01 and 0.80 ± 0.4 nm2/µs, respectively, compared to Dtr = 0.34 ± 0.01 nm2/µs for RP in the cytoplasmic condensates.
RP and POSL concentrations were varied systematically, while the concentration of POSS was kept constant and the number of CRW particles was adjusted to maintain a constant total molecular volume (Supplementary file 2). Cluster size distributions were extracted (Figure 2—figure supplement 3) and the fraction of tRNA and RP in the large clusters was determined (Figure 2). Some degree of clustering occurs at all concentrations, but condensation requires that a significant fraction of particles is found in the largest clusters. Based on a criterion that at least half of the particles are found in one or few large clusters, tRNA and RP condensation occurs for [POSL]>100 μM (Figure 2).
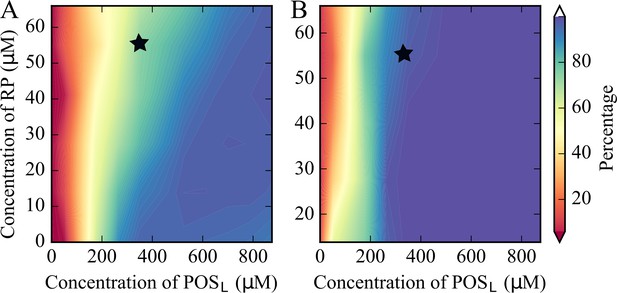
Percentage of tRNA (A) and RP (B) in largest clusters in coarse-grained simulations of the five-component model system as a function of [RP] and [POSL].
The black star indicates the conditions that match the cytoplasmic model. tRNA condensation is a phase separation process.
Increasing [RP] reduces the amount of tRNA in the tRNA condensates and effectively raises the critical POSL concentration above which tRNA forms condensates (Figure 2). This can be understood from competition for POSL. tRNA only interacts significantly with POSL (Figure 2—figure supplement 4) and needs POSL to form condensates, whereas RP interacts with both POSS and POSL (Figure 2—figure supplement 5) and therefore draws POSL from tRNA condensates (Figure 2—figure supplement 6). For [POSL]>500 μM, the fraction of tRNA particles in the tRNA condensates is relatively constant (Figure 2). However, the number of POSL particles in the condensates increases as the total [POSL] increases (Figure 2—figure supplement 6). This results in larger clusters and lower effective [tRNA] in the condensates at the highest values of [POSL] (Figure 2—figure supplement 7). The effect of increasing [RP] is again a depletion of POSL in the tRNA condensates, so that [tRNA] in the condensates increases with [RP] for a given value of [POSL] (Figure 2—figure supplement 7).
In the simulations described so far, the total volume fraction of the system was kept constant by reducing the crowder (CRW) concentration as [POSL] and [RP] increased. Therefore, the decrease in [tRNA] inside the condensates with increasing [POSL] could be due to reduced crowder interactions in the condensate environment. To test this further, we reduced [CRW] without changing [POSL]. Reduced [CRW] also led to reduced [tRNA] in the condensate, but the effect is much smaller than when [CRW] is reduced along with an increase in [POSL] (Figure 2—figure supplement 8).
In order to construct phase diagrams, simulations of the five-component model phases were carried out at a range of temperatures for selected values of [RP] and [POSL]. Cluster size distributions were extracted (Figure 3—figure supplements 1–3) and the volume fractions of tRNA in dilute and condensed phases as a function of temperature were determined based on the number of tRNA outside and inside the largest tRNA clusters. The volume of the condensed phase containing the largest tRNA cluster was calculated as described above. The resulting curves (Figure 3) show the typical features of phase diagrams with phase coexistence below critical temperatures Tc of 400–535 K. In the absence of ribosomes, that is, [RP]=0, an increase in [POSL] lowers Tc and narrows the two-phase regime (Figure 3C). This is consistent with reentrant phase behavior expected for complex coacervation of a binary mixture. However, in the presence of ribosomes, that is, [RP]=55 μM, Tc increased at the same time as the two-phase regime narrowed with increasing [POSL] (Figure 3D). Moreover, when [POSL]=180 μM, near the minimum needed for PS, an increase in [RP] slightly decreased Tc (Figure 3A/E), whereas at a higher concentration, that is, [POSL]=880 μM, Tc increased with increasing [RP] up to a maximum at 55 μM before decreasing (Figure 3B/E). These observations reflect competition between ribosomes and tRNA for interactions with POSL and more generally highlight the effects of a complex interplay between interactions in non-binary mixtures that are more representative of biological environments than simple binary mixtures.
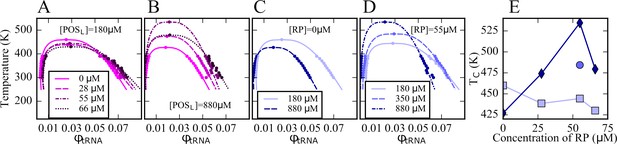
Phase diagrams for tRNA with [POSL]=180 μM and varying RP concentrations (A); with [POSL]=880 μM and varying RP concentrations (B); with [RP]=0 at two [POSL] concentrations (C); and with [RP]=55 μM and varying POSL concentrations (D); critical temperatures as a function of [RP] at [POSL]=180 μM (squares), at [POSL]=880 μM (diamonds), and at [POSL]=350 μM (sphere) (E).
The volume fractions of tRNA in the dilute and condensed phases were obtained based on the number of tRNA particles in the dilute and condensed phases normalized by the respective volumes of the two phases (see Text). Lines in A–D were fitted according to Equations 9 and 10.
Phase separation in experiments for binary mixtures of globular RNA and proteins
The results presented so far have focused on multi-component systems that were modeled to reflect the density and distribution of particle sizes and charges in cytoplasmic environments. A key prediction is that PS due to complex coacervation may occur for a wide range of nucleic acids and positively charged proteins simply based on electrostatic complementarity. To test this idea experimentally, we now turn to binary mixtures of globular RNA and positively charged proteins. We focused on the 47-nucleotide J345 Varkud satellite ribozyme RNA, that folds into an approximately globular shape (Bonneau and Legault, 2014) and that was mixed at high concentration with common proteins with positive charges and varying sizes for which we may expect PS: myoglobin (q = +2), trypsin (q = +6), lysozyme (q = +8), lactate dehydrogenase (LDH; q = +4), and alcohol dehydrogenase (ADH; q = +8). Bovine serum albumin (BSA; q = −17, r = 2.58 nm) was added as a control, for which condensate formation is not expected due to its negative charge.
Imaging via confocal microscopy of dye-labeled RNA (Figure 4 and Figure 4—figure supplements 1–6) shows well-defined fluorescent clusters for mixtures of RNA with trypsin, ADH, lysozyme, and LDH, but not for RNA with myoglobin or BSA. The background fluorescence varies significantly with protein. It is especially high for the mixtures with LDH, suggesting that only a fraction of RNA is participating in the condensates and a larger fraction of RNA remained in the dilute phase.
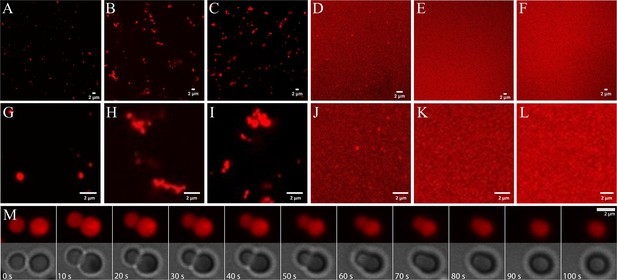
Phase separation in mixtures of J345 RNA at 0.45 mM and various globular proteins at 0.35 mM from confocal microscopy of labeled RNA: trypsin (A; G), ADH (B; H), lysozyme (C; I), LDH (D; J), myoglobin (E; K), BSA (F; L).
Time lapse of droplet merging in RNA-trypsin mixture from fluorescence and bright-field microscopy imaging (M).
Individual condensates are relatively small, and many appear to have sizes near or below the diffraction limit of the microscope. For RNA-trypsin mixtures, we clearly observe single droplet-shaped condensates of varying sizes that follow roughly an exponential distribution (Figure 4—figure supplement 7). We note that the concentration of Cy3-labeled RNA is only 8 µM, corresponding to 1 in 56 RNA at 0.45 mM total RNA concentration. Therefore, the fluorescent images in Figure 4 are biased toward clusters that contain at least 50 RNA molecules, whereas smaller clusters are imaged incompletely. RNA-LDH condensates appear similar but we did not attempt a quantitative size analysis due to the high background fluorescence of the RNA-LDH sample. Diffusing droplets in the RNA-trypsin mixture merge over the course of 1 min when they come into proximity (Figure 4M and Videos 2 and 3), indicative of liquid behavior inside the condensates.
Merging of trypsin-RNA liquid condensate droplets.
Video of two representative examples of liquid droplet dynamics in trypsin-RNA mixtures with J345 RNA at 0.45 mM and proteins at 0.35 mM from confocal microscopy of fluorescent-labeled RNA (left) and corresponding bright-field imaging (right). Time evolution is accelerated 25x (i.e. the movies correspond to about 100 s in real time).
Merging of trypsin-RNA liquid condensate droplets.
Video of two representative examples of liquid droplet dynamics in trypsin-RNA mixtures with J345 RNA at 0.45 mM and proteins at 0.35 mM from confocal microscopy of fluorescent-labeled RNA (left) and corresponding bright-field imaging (right). Time evolution is accelerated 25x (i.e. the movies correspond to about 100 s in real time).
For other proteins (lysozyme and ADH), we found more complex condensate morphologies (Figure 4), where smaller condensates associate to form larger, irregular-shaped condensates without merging as seen for RNA-trypsin condensates. This suggests that the condensates with these proteins are less liquid-like, although the exact nature of the condensates not involving trypsin is unclear.
To further study the particle size distributions, we carried out dynamic light scattering (DLS) analysis on RNA/lysozyme and RNA/trypsin samples (Figure 5, Figure 5—figure supplements 1–2, and Table 1). The light scattering correlation functions indicate a polydisperse sample that is dominated by very long correlation times up to 1 s (Figure 5). Those long correlation times theoretically correspond to macroscopic-size particles (Stetefeld et al., 2016), but since no such particles were readily visible in the sample, we may conclude that a significant fraction of condensates exhibited very slow diffusion due to surface adsorption. From the correlation function at shorter times, multi-exponential fits suggest particles in two size regimes for RNA-trypsin and in three regimes for RNA-lysozyme. In both cases, the data indicate the presence of 10 nm-scale particles that are consistent with oligomer-size clusters of RNA and protein molecules. Such small clusters between RNA and/or proteins are expected to be present in the dilute phase due to transient associations (Nawrocki et al., 2017; Yildirim et al., 2018; Barhoum and Yethiraj, 2010; Kowalczyk et al., 2011). In both, RNA-trypsin and RNA-lysozyme sample, the DLS analysis suggests the presence of µm-size particles (somewhat smaller for trypsin than for lysozyme). In addition, the DLS data indicate the presence of particles at the light microscopy diffraction limit, around 300 nm, for the RNA-lysozyme system but not for RNA-trypsin mixtures. In fact, the DLS results are qualitatively consistent with the microscopy images and provide additional insights into the particle size distributions at and below the light diffraction limit. However, an exact quantitative interpretation of the DLS results is challenging due to the polydispersity and dynamic nature of our samples and for that reason we also did not attempt to quantify what fraction of particles would be expected in the different size regimes.
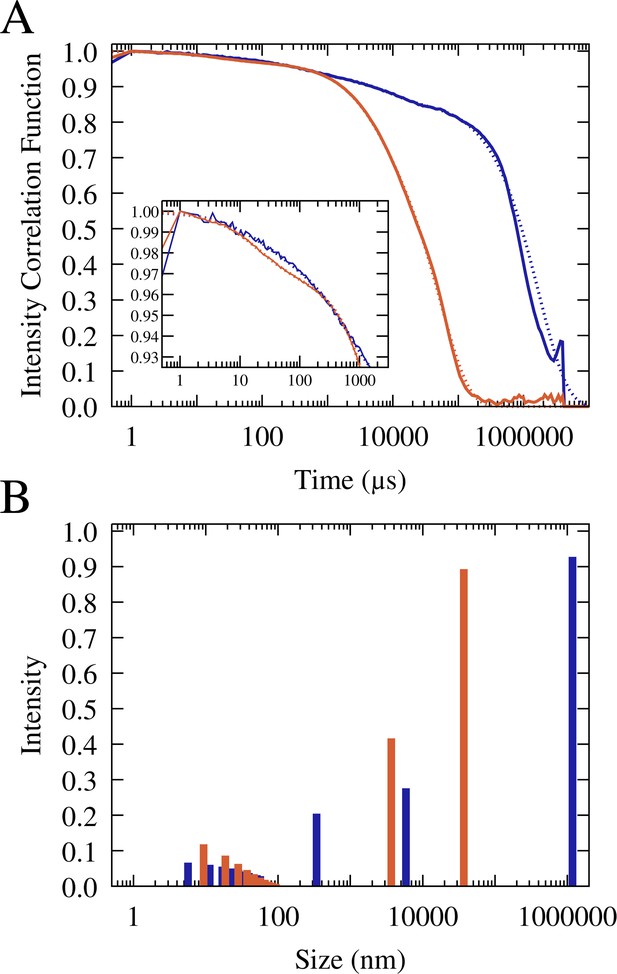
Normalized and averaged scattering intensity correlation functions from triplicate dynamic light scattering experiments of mixtures of 0.1 mM J345 RNA with 0.166 mM trypsin (orange) and 0.4 mM RNA with 0.675 mM lysozyme (blue) (A).
Scattering intensity as a function of particle size from multi-exponential fits to the correlation functions (shown as dotted lines in A) for trypsin (orange) and lysozyme (blue) (B).
Multi-exponential fits of dynamic light scattering correlation functions.
| System* | Clusters | Size 1 | Size 2 | Size 3 | Size 4 | χ2 | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Dc (nm) | ac | tc | D1 (nm) | a1 | D2 (nm) | a2 | D3 (µm) | a3 | D4 (µm) | a4 | *10−3 | |
| Lysozyme #1 | 6.8 | 0.076 | 9.4 | 314.5 | 0.197 | 6061 | 0.309 | 1037.0 | 0.919 | 0.362 | ||
| Lysozyme #2 | 4.3 | 0.085 | 10.5 | 325.4 | 0.240 | 5416 | 0.300 | 730.4 | 0.908 | 0.91 | ||
| Lysozyme #3 | 4.0 | 0.045 | 21.7 | 270.0 | 0.160 | 2585 | 0.163 | 17.6 | 0.190 | 28,373.6 | 0.948 | 0.13 |
| Lysozyme avg. | 5.6 | 0.073 | 10.4 | 339.4 | 0.204 | 5848 | 0.275 | 1184.7 | 0.927 | 0.16 | ||
| Trypsin #1 | 7.6 | 0.129 | 5.0 | 2544 | 0.345 | 30,167 | 0.921 | 1.6 | ||||
| Trypsin #2 | 2.7 | 0.051 | 186,625 | 2003 | 0.297 | 38,323 | 0.942 | 1.46 | ||||
| Trypsin #3 | 2.4 | 0.055 | 106,796 | 5210 | 0.575 | 46,527 | 0.801 | 1.05 | ||||
| Trypsin avg. | 9.3 | 0.162 | 3.2 | 3680 | 0.417 | 36,967 | 0.893 | 2.31 | ||||
-
*All systems are mixtures between protein and J345 RNA.
To map out a phase diagram, we prepared RNA-trypsin mixtures at various experimentally feasible RNA and protein concentrations. PS required a minimum protein concentration, for example with [RNA]=100 µM, PS was found with [trypsin]=150 µM but not with [trypsin]=50 µM (Figure 6—figure supplement 1). At the same time, PS was lost when RNA concentrations were too high. The resulting phase diagram based on confocal microscopy imaging is shown in Figure 6 in comparison with results from theory that are discussed below.
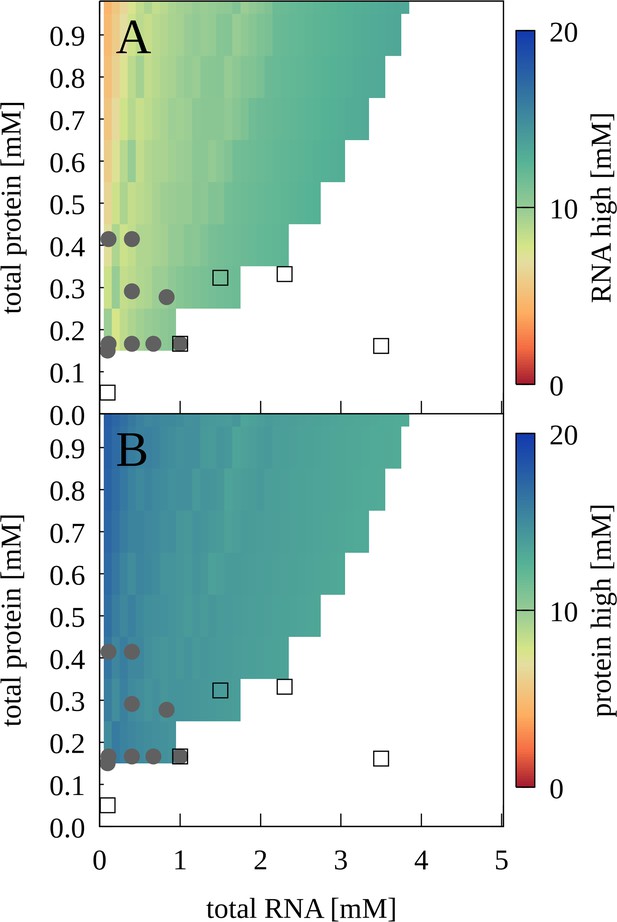
Phase separation for mixtures of J345 RNA and trypsin as a function of total protein and RNA concentrations from experiment and theory.
Grey filled circles indicate concentrations for which phase separation was observed experimentally based on confocal microscopy; empty squares indicate concentrations for which microscopy imaging did not show phase separation. Colors indicate predicted concentrations from theory for RNA (A) and proteins (B) in the condensed phases. No phase separation is predicted for white areas.
Förster resonance energy transfer (FRET) experiments also showed a significant increase in FRET efficiencies from 50 to 150 µM (Figure 7A). The comparison between the microscopy and FRET results furthermore establishes that RNA condensates at this RNA concentration can be recognized by FRET efficiencies above 0.26, whereas lower values may indicate a disperse phase. The gradual increase in FRET efficiencies from 0.24 to 0.26 upon increase of trypsin concentrations from 0 to 50 µM is interpreted to result from increasing non-condensate cluster formation (see cluster size distributions in Figure 2—figure supplement 3 at [RP]=0 with increasing protein concentration). However, as in the confocal microscopy experiments, the low concentration of fluorescence-labeled RNA limits the detection of very small clusters where only one or zero of the RNA would be labeled. The FRET results are compared with theoretical predictions (Figure 7B) as detailed below.
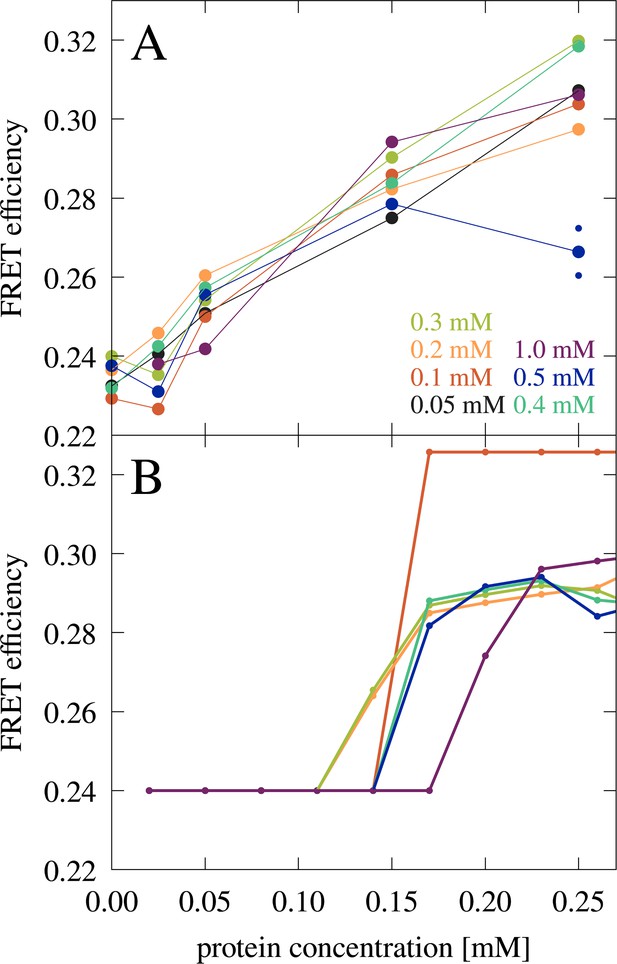
FRET efficiency in mixtures of J345 RNA with trypsin as a function of protein concentration at different RNA concentrations (as indicated by color).
The average of two measurements is shown for 0.25 mM protein and 0.5 mM RNA concentrations with smaller points indicating individual measurements. (A) FRET efficiency estimated from the fraction of RNA in the condensed phase from theory (B). In every measurement, the concentration of Cy3- and Cy5-labeled RNA is constant, 8 µM and 42 µM, respectively.
We applied circular dichroism (CD) and nuclear magnetic resonance (NMR) spectroscopy with the goal of examining whether the proteins and RNA retain their folded states upon condensate formation. The CD spectra in Figure 4—figure supplement 8 show that there is no substantial change in the shape of the spectrum of trypsin in the presence of the RNA from 225 to 250 nm, which would be expected if the protein had unfolded, as a random coil spectrum has essentially no ellipticity in this wavelength range and the spectrum. The key feature of the RNA spectrum, the broad peak at 250–290 nm, is also retained in the mixture. In fact, the spectrum of the trypsin-RNA mixture appears to be simply a linear combination of the spectra of each of the components measured separately.
NMR spectroscopic analysis of RNA-trypsin and RNA-lysozyme samples at PS-inducing concentrations focused on the structure of the RNA. We observed the characteristic 1H spectrum of a solution containing only J345 RNA that matches previously matched spectra for the same structure (Bonneau and Legault, 2014; Figure 4—figure supplement 9). In the presence of proteins, the characteristic peaks were retained at the same positions, although with greatly attenuated intensities (Figure 4—figure supplement 9). This was interpreted to mean that only a fraction of RNA remained sufficiently dynamic to achieve rotational averaging via molecular tumbling. From comparing the signal-to-noise ratios, we estimate that about 80% of the RNA is not visible in the RNA-lysozyme sample and 90% is invisible in the RNA-trypsin sample. Since the majority of RNA is expected to be found in the condensates, this suggests that rotational diffusion of individual RNA molecules in the condensates is retarded significantly because the condensates themselves are too large (>100 nm) to tumble on time scales allowing NMR signals to be observed (<100 ns). Moreover, if one assumes that only RNA in the dilute phases remains visible in NMR spectroscopy, the experiments provide an estimate of the fraction of RNA in the dilute vs. condensed phases, that is 20:80 in the presence of lysozyme and 10:90 in the presence of the trypsin for the concentrations studied here. Unfortunately, that also implies that there is no information about the structure of RNA inside the condensates from these experiments.
Phase separation of RNA and proteins described by simulations and theory
To compare with the experimental findings, we carried out CG simulations again with the model described above but for binary mixtures of spherical particles equivalent in size and charge to the experimentally studied systems, that is, J345 RNA (q=-46, r = 1.47 nm), myoglobin (q = +2, r = 1.64 nm), trypsin (q = +6, r = 1.81 nm), lysozyme (q = +8, r = 1.54 nm), lactate dehydrogenase (LDH; q = +4, r = 2.68 nm), alcohol dehydrogenase (ADH; q = +8, r = 2.79 nm), and bovine serum albumin (BSA; q = −17, r = 2.58 nm). We also tested a spherical particle equivalent to cytochrome C (q = +11, r = 1.45 nm) which was not studied experimentally because of heme absorption. We observed the formation of condensates at sufficiently high-salt concentrations. With κ = 0.7 (about 20 mM salt), condensates formed with lysozyme, trypsin, LDH, and ADH, but not with cytochrome C, myoglobin, or BSA (Figure 8). Very similar results were also found with an alternative effective charge model (according to Equation 6) as shown in Figure 8—figure supplement 1.
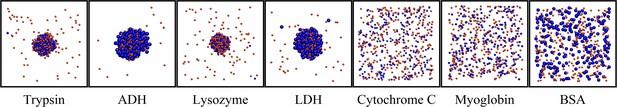
Snapshots after 1 ms for binary RNA-protein mixtures at T = 298K, with κ = 0.7 and using effective charges according to Equation 5.
[RNA]=0.493 mM and [protein]=0.350 mM. Orange and blue spheres show RNA and proteins, according to size. Concentrations inside the condensates were [RNA:lysozyme]=20.2:20.2 mM; [RNA:trypsin]=16.5:15.2 mM; [RNA:LDH]=9.6:7.2 mM; [RNA:ADH]=9.5:6.7 mM.
The simulation results qualitatively match the experimental results in terms of which proteins promote PS. Moreover, the fraction of RNA in the dilute phase is higher with lysozyme than with trypsin (32% vs. 26–27% using Equation 5 or Equation 6 from averages over the last 100 µs) in qualitative agreement with the estimates from the NMR experiments. We note that an overall larger fraction of RNA is expected in the dilute phase in the simulations due to an excess concentration of RNA (0.439 mM) compared to the protein concentration (0.350 mM), whereas concentrations of RNA and protein were equal in the NMR experiments (0.150 mM). However, the scale of the simulations is too small to directly compare the condensate sizes with the experimental size distributions.
To generate more extensive phase diagrams, a theoretical model was developed based on the CG simulations. Briefly, the model approximates the chemical potential for either RNA or proteins in condensed and dilute phases based on a decomposition into enthalpy and entropy: . The enthalpy is determined from convoluting the coarse-grained interaction potential U(r) (Equation 3) with radial distribution functions of RNA-RNA, RNA-protein, and protein-protein interactions in the condensed and dilute phases extracted from CG simulations and scaled by particle densities ρ:
The entropy was estimated from the ratio of particle densities ρ between the entire system and either the dilute or condensed phase:
Solutions with respect to the concentrations of protein and RNA in dilute and condensed phases were determined numerically under the conditions that µcondensed = µdilute for either RNA, protein, or both, and that molecular volume packing fractions did not exceed maximum packing densities. Total free energies were then calculated, taking also into account mixing entropy contributions between RNA and protein particles. PS was predicted based on the solution with the lowest free energy.
The theoretical approach is essentially a variation of Voorn-Overbeek theory (Overbeek and Voorn, 1957) for spherical particles. While this theory has seen numerous applications, especially to polyelectrolyte fluids (Priftis et al., 2014; Spruijt et al., 2010), the specific model described here emphasizes an interaction potential that is parameterized based on atomistic simulations of biological macromolecules and that was further tuned to match experimental data. Therefore, the theory is expected to make predictions that are more relevant for globular biological macromolecules than previous studies.
In developing the theory, we found that using the alternative effective charge model according to Equation 6 (Figure 1—figure supplement 8) results in better agreement between theory and experiment and therefore we used this model here. We also use a slightly different Debye-Hückel screening term, that is, κ = 1.17, which gave better agreement between theory and experiment.
Application of the theory predicts that PS should occur for a wide range of protein radii and charges as long as proteins are large enough and carry sufficiently positive charge (Figure 9). More specifically, radius/charge combination corresponding to lysozyme, trypsin, LDH, and ADH are predicted to lead to PS as in the experiments and CG simulations. The radius and charge corresponding to myoglobin is just outside the PS region (Figure 9) again consistent with the lack of PS in the experiment and simulations. The theory also predicts PS for cytochrome C, for which PS was not seen in the simulations.
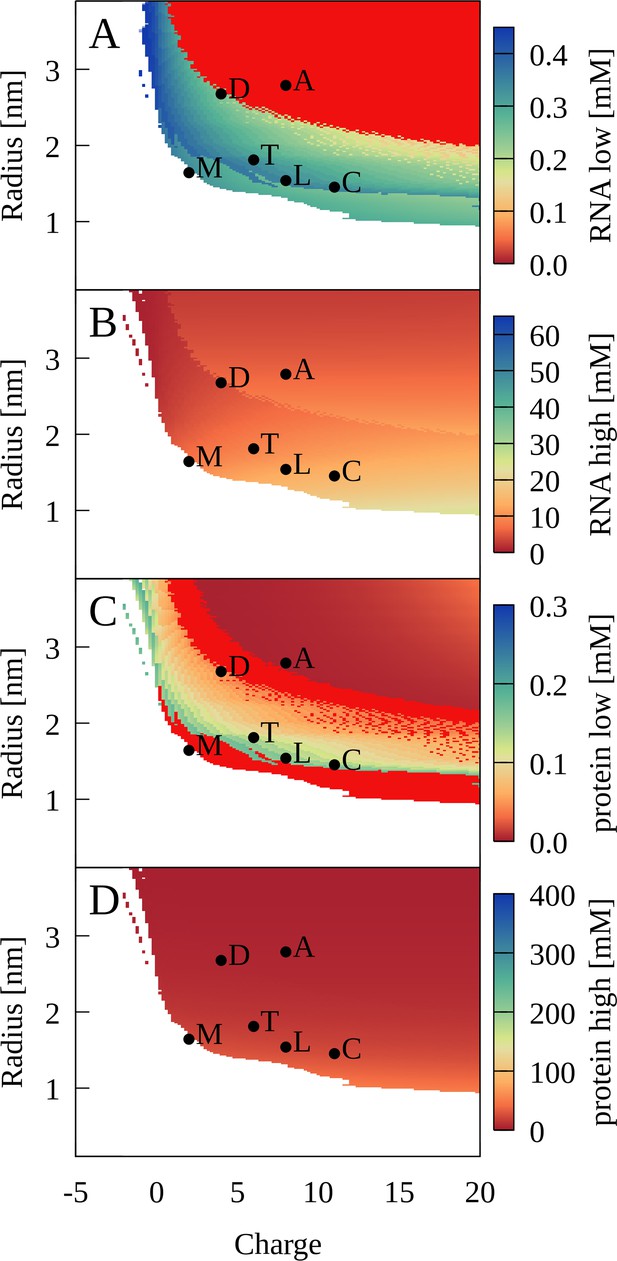
Phase separation for binary RNA-protein mixtures as a function of protein charge and radius from theory.
Colors show [RNA] (A, B) and [protein] (C, D) in dilute (A, C) and condensed (B, D) phases. Red indicates zero concentration. [RNA]=0.45 mM, [protein]=0.35 mM, κ = 1.17, and T = 298 K. Corresponding properties for proteins are denoted as follows: myoglobin (M); trypsin (T); lysozyme (L); cytochrome C (C); LDH (D); ADH (A).
The theory reproduces an expected temperature dependence of PS with protein-dependent critical maximal temperatures (Figure 8—figure supplement 2). The electrostatic nature of PS also suggests that changes in salt concentrations would affect the findings and the results are indeed sensitive to the value of κ. However, the theoretical treatment is too limited due to the mean-field nature of the Debye-Hückel formalism to make meaningful predictions of salt effects. More specifically, the model is only valid for low ionic strengths and ignores entropic consequences of ion partitioning between condensed and dilute phases that are an important contribution to PS in complex coacervates (Vis et al., 2015).
Using the theory, we constructed concentration-dependent phase diagrams that can be compared with experiment. Figure 6 shows the prediction of the two-phase region for RNA-trypsin in good agreement with the experimental data. Figure 9—figure supplements 1–6 show the phase diagrams for all proteins studied here over a wider range of concentrations. All phase diagrams exhibit reentrant behavior with minimal and maximal protein and RNA concentrations as expected for complex coacervates. It should be noted, though, that the full range of concentrations cannot be realized in practice for all systems due to limited solubilities.
Predictions from the theory also allowed a quantitative interpretation of the FRET experiments. Using the predicted fraction of RNA in the condensates for the RNA-trypsin mixtures at different RNA and protein concentrations (Figure 9—figure supplement 7), FRET efficiencies were estimated (Figure 7B). The theoretical predictions qualitatively reproduce the experimental data with an onset of increased FRET efficiencies due to condensation. Moreover, the gradual increase in FRET efficiencies after condensates form is predicted from a growing number of RNA in the condensed phase as protein concentration increases.
Discussion
This study presents a general view on charge-driven biomolecular PS supported by simulation, theory, and experiments. More specifically, we report a potential for PS between negatively charged RNA and positively charged proteins without requiring polymer-character of either component or specific binding interactions. Our simulations and the theoretical model are based on isotropic spheres, whereas experimental validation is based on a compact, approximately globular RNA and a variety of globular proteins that are not known to specifically interact with RNA. This implies that PS may be a very general phenomenon in biological cells depending on the concentrations, charge, and size distribution of available nucleic acid and protein components. In fact, our simulations of a bacterial cytoplasm provide examples of separately forming tRNA-protein and ribosome-protein condensates involving a variety of proteins in a cytoplasmic environment. Separate condensates of nucleic acids with different charge and size could have important implications for the role of PS in vivo.
The idea of strong complementary electrostatic interactions playing a major role in PS via complex coacervate formation is well-established for a variety of different molecules (Sawyer et al., 2019; de Kruif et al., 2004; Fay and Anderson, 2018; Cummings and Obermeyer, 2018; Michaeli et al., 1957; Mattison et al., 1995) and also for PS involving biomolecules (Ghosh et al., 2019). While almost all the LLPS studies to-date involve polymers and in particular IDPs (Dignon et al., 2018a), there are also examples in the literature that discuss PS involving folded proteins (Sanders et al., 2020; Aumiller et al., 2016; Banani et al., 2016; Li et al., 2012; Banjade and Rosen, 2014; Conicella et al., 2020). In most of those cases, the ability to form condensates is generally ascribed to specific multi-valent interactions and evidence for a more generic electrostatic-only mechanism are only just beginning to emerge (Cummings and Obermeyer, 2018; Sanders et al., 2020). The results presented here provide evidence for a more general principle that does not require flexible polymers, specific interaction sites, or specific secondary structures (Conicella et al., 2020). The central principle is simply electrostatic complementarity at the molecular level, but a more generalized concept of multi-valency is implicitly assumed. Isotropic spheres without any directional preference for interactions are in fact maximally multi-valent, limited only by the excluded-volume interactions between the binding partners. On the other hand, globular proteins with basic amino acids distributed widely across their surface and diffuse positive electrostatic potentials over most of the molecular surface (Figure 8—figure supplement 3) are effectively poly-valent particles with respect to interactions with nucleic acids. The key insight from this study is that proteins not known to interact specifically with nucleic acids under dilute conditions may form condensates with nucleic acids, if the proteins are present at sufficient amounts, simply based on a principle of generic poly-valency and an overall charge attraction.
Our study suggests that size and charge are essential determinants of PS between RNA and proteins. Favorable condensates require optimal packing and a balance of attractive and repulsive interactions between oppositely charged RNA and protein particles. Figure 1—figure supplement 9 shows a snapshot from the cytoplasmic system illustrating how such packing may be achieved. The optimal balance depends on the size of the RNA particles: Larger proteins are required for the smaller RNA molecules to phase separate, whereas smaller proteins allow the larger ribosomal particles to phase-separate (Figure 1—figure supplement 5). This can be seen more clearly in the five-component model system, where a relatively modest reduction in the radius of the larger positively charged particle leads to a loss of close tRNA contacts (Figure 1—figure supplement 10), therefore preventing condensate formation. The theoretical model for binary RNA-protein mixtures also predicts a minimum protein radius for PS, at least at lower charges (Figure 9). Myoglobin is outside the predicted range and although it has a net-positive charge, PS was not observed in the experiment at protein concentrations below the RNA concentrations (Figure 4) consistent with the theory. The sensitivity to matching size and charge between the RNA and proteins suggests at least a partial explanation for the observation of separate condensates for tRNA and RP in the simulations of the cytoplasmic model systems.
The total concentration of the protein is another determinant for PS. Simulations and theory predict minimum protein concentrations depending on the protein charge and size around 0.05 mM or more (Figure 9—figure supplements 1–6). For trypsin, this was validated experimentally via microscopy and FRET spectroscopy (Figures 6 and 7). While many cellular proteins may not be present individually at such high concentrations, our cytoplasmic model shows that a heterogeneous mixture of similar-sized and similar-charged proteins may promote PS equally well. At the lower end, the RNA concentration appears to be a less critical factor for observing PS, although a larger amount of RNA allows more numerous and larger condensates to form, assuming that there is enough protein available, at least until reaching a critical RNA concentration beyond which PS is not favorable anymore. In binary mixtures, this is simply a question of the total protein concentration. In the heterogeneous cytoplasmic model, we found competition for the larger positively charged proteins by the ribosomes forming their condensates to be another factor affecting tRNA condensate formation that would need to be considered in cellular environments (Figure 2—figure supplement 7).
Since electrostatics is a major driving force of the PS described here, changes in salt concentration are expected to alter the tendency for PS. The theory applied here is not well-suited to examine variations in the salt concentration. At the same time, there is only a limited range of decreased salt conditions that can be applied before either the RNA or the protein structures become destabilized. Therefore, we could not yet develop an accurate quantitative understanding of how salt effects may affect charge-driven phase separation. This topic will have to be deferred to future studies.
A significant interest in PS in biology is related to liquid-state condensates. Such condensates would maintain the dynamics that is necessary for many biological processes as opposed to dynamically retarded gels or amorphous clusters. The simulations suggest that the condensates retain significant dynamics based on calculated self-diffusion rates, although there are serious limitations on diffusion estimates from CG simulations, especially in the absence of hydrodynamic interactions (Ando and Skolnick, 2010). In experiment, we find evidence of liquid-like behavior for condensates formed in RNA-trypsin mixtures, but the dynamic properties of RNA or proteins in other RNA-protein condensates are less clear. As another data point, the NMR spectroscopy results also suggest significant retardation of diffusional dynamics inside the condensates.
Although there are some limitations in the current study that will need to be revisited in future studies to gain a more detailed understanding of the more universal PS between RNA and proteins described here, the main advantage of the CG models and theory is that its simplicity allowed us to explore the large spatial scales and long-time scales that can predict phase behavior on experimentally accessible scales. The CG models were parameterized based on high-resolution atomistic simulations of concentrated protein solutions, these models lack all but the most basic features of biological macromolecules. Increased levels of realism could be achieved without too much additional computational cost via patchy particles (Nguemaha and Zhou, 2018), whereas higher-resolution in the form of residue-based coarse-graining (Dignon et al., 2018a) to explore the effects of shape anisotropy and inhomogeneous charge distributions across RNA and protein surfaces is in principle attainable but computationally much more demanding.
The cytoplasmic model described here is a first step toward modeling biologically relevant environments, but leaves out DNA, membranes, and other cellular structures such as the cytoskeleton. The CG version of the cytoplasmic model furthermore neglects metabolites, whereas the representation of macromolecules as spheres is clearly an oversimplification, especially for more flexible and irregularly shaped molecules such as mRNAs or proteins with significant intrinsic disorder or internal dynamics. Future studies will aim to include the missing factors to examine how important such additional details are for modulating phase separation processes in vivo.
Finally, we expect that further insights could be gained from atomistic simulations of RNA-protein clusters initiated from configurations in the CG simulations to better understand the detailed molecular interactions stabilizing the condensates. On the experimental side, we only focused on RNA without visualizing protein condensation. Moreover, there is a need to follow up on this work with in vivo studies to establish how ubiquitous the condensates described here are under cellular conditions.
Conclusions
We report phase separation of RNA and proteins based on a universal principle of charge complementarity that does not require polymers or multi-valency via specific interactions. The results are supported by coarse-grained simulations, theory, and experimental validation via microscopy, FRET, and NMR spectroscopy as well as DLS experiments. Condensate formation depends on concentration, size, and charge of the proteins but appears to be possible for typical RNA and common proteins. Simulation results, furthermore, suggest that such phase separation may occur in heterogenous cellular environment, not just between tRNA and cellular proteins but also, in separate condensates, between ribosomes and proteins. Further computational and experimental studies are needed to gain more detailed insights into the exact molecular nature of the condensates described here.
The larger implication of the work presented here is that charge-driven phase separation appears to be a broad phenomenon in biology, particularly because intrinsically disordered proteins and disordered RNA are not required. As a result, cellular cytoplasms could be phase-separated extensively. The observation that tRNA could condense and co-locate near ribosomes suggests a mechanism in which the rate of protein translation is increased because the diffusional wait time for the correct tRNA arriving at the ribosome is decreased. It remains to be explored through in vivo experiments how widely charge-driven phase separation may present itself in cellular environments and what additional factors may modulate it.
Materials and methods
Coarse-grained model
Request a detailed protocolCG simulations were run using a modified version of a previously introduced colloid-type spherical model (Mani et al., 2014). In this model, pair interactions consist of a short-range 10–5 Lennard-Jones potential and a long-range Debye Hückel potential according to:
where rij is the inter-particle distance, σij is the distance between particles at which the potential is zero, ε is the strength of short-range attraction, Aij+A0 describes attractive or repulsive long-range interactions, and κσij is the Debye-Hückel screening length. Only Aij and σij vary between different particles according to charge and size.
The model was initially parameterized from previously published all-atom simulations of homogeneous mixtures of chicken villin headpiece (‘villin’) (Nawrocki et al., 2017) and subsequently validated with heterogeneous mixtures of protein G, villin, and ubiquitin (Nawrocki et al., 2019a) as summarized in Table 2.
Simulation systems for coarse-grained model validation.
| System | Villin | Protein G | Ubiquitin | Box (nm) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Volume percentage | G/L | mM | Np* | G/L | mM | Np* | G/L | mM | Np* | |
| 5% | 9.7 | 2.3 | 5 | 14.3 | 2.3 | 5 | 19.8 | 2.3 | 5 | 15.3 |
| 10% | 19.0 | 4.5 | 10 | 28.2 | 4.5 | 10 | 39.0 | 4.5 | 10 | 15.4 |
| 30% | 57.9 | 13.8 | 30 | 85.7 | 13.8 | 30 | 118.6 | 13.8 | 30 | 10.6 |
-
*Number of proteins.
A common value of ε = 4.0 kJ/mol was used for all particles in the short-range 10–5 Lennard-Jones potential. Particle size was taken into account by first determining the radii ri of spheres with equivalent volumes to the atomistic molecular volumes of a given macromolecule or complex (see Supplementary file 1 for molecules in the cytoplasmic model system). Lennard-Jones parameters σi were obtained from the radii ri according to:
Pairwise parameters σij were calculated as σij = σi + σj.
In the long-range Debye-Hückel type potential, a common value of A0 = 3.0 kJ/mol was used to reflect effective repulsion between charge-neutral, but still polar molecules due to solvation effects. Net charges led to additional repulsive or attractive contributions.
The nominal net charge of a given molecule was converted to effective charges to account for counterion condensation around highly charged macromolecules (Manning, 1978). We distinguish here effectively bound ions that lead to an effectively reduced charge vs. ions that remain mobile in solution and give rise to Debye screening as described below. Generally, the effective charge remains close to nominal charges for small charges, but for highly charged molecules, in particular negatively charged nucleic acids and nucleic acid complexes such as the ribosome, the effective charge is reduced significantly (Diehl and Levin, 2004; Wishnia and Boussert, 1977; Trylska et al., 2004). Charge neutralization is more pronounced with divalent ions such as Mg2+ vs. monovalent ions such as Na+ or K+ (Diehl and Levin, 2004; Templeton and Elber, 2018; Yoo and Aksimentiev, 2012). But the amount of Mg2+ ions in biological systems is limited and typically not high enough to neutralize the charge of all the nucleic acids so that additional charge neutralization by monovalent ions remains a significant factor (Akanuma et al., 2014).
Here, we propose the following two expressions to obtain effective charges:
Both empirical formulae give effective charges close to nominal charges for molecules with small charges and highly reduced charges for molecules with large formal charges (Figure 1—figure supplement 8). For a DNA molecule with a nominal charge of −45, atomistic MD simulations suggest effective charges of −10 to −20 under the assumption that ions within 1 nm from the solute surface are effectively bound (Templeton and Elber, 2018; Yoo and Aksimentiev, 2012); at the other end, effective charges between −100 and −800 are estimated for ribosomal particle with a nominal charge of about −4000 based on colloid models (Diehl and Levin, 2004) or electrostatic potential calculations (Trylska et al., 2004), assuming a mixture of divalent and monovalent ions is involved in neutralization. Equations 5 and 6 are both consistent with these estimates. Equation 5 was used initially and screens smaller charges less and larger charges more strongly compared to Equation 6 which was adopted after adjusting the theory to better match experimental results. Neither expression considers ionic concentration as counterion condensation does not depend strongly on concentration (Yoo and Aksimentiev, 2012). Moreover, negatively and positively charged solutes are treated in the same manner even though the binding strength of biological anions (Cl-) and cations (K+, Na+, Mg2+) to oppositely charged macromolecules may be asymmetric. However, since highly positively charged macromolecules are uncommon, this assumption may not have significant consequences for the systems studied here.
The effective charges calculated either via Equation 5 or Equation 6 were then converted to Ai values:
Pairwise values were determined as Aij=Ai*Aj and the factor ¾ was determined by parameterization against the atomistic MD simulations.
The Debye screening length in Equation 3 is κσij, that is, it depends on particle size as in the original model by Mani et al., 2014 in order to better model screening interactions between particles of very different sizes with screened charges that are mostly near the surface. This complicates interpretation of κ in terms of specific salt concentrations. However, as an illustration one may consider a typical smaller protein or RNA with σii of 3 nm where κ = 0.5, 1.0, and 1.5 would correspond to monovalent ion concentrations of 40, 10, and 5 mM, respectively. Note, that these ion concentrations reflect excess ion concentrations after subtracting condensed counterions as those are accounted for in the effective charges according to Equation 5 or 6. Therefore, total ion concentrations in experiment corresponding to a given value of κ in our model should be significantly higher by factors of 2–10 depending on the charges of the considered macromolecules.
Coarse-grained molecular dynamics simulations
Request a detailed protocolMD simulations of the CG model were run up to 1 ms using OpenMM (Eastman et al., 2017) on GPU machines. The interaction potential from Equation 3 was implemented as a custom non-bonded interaction potential via OpenMM’s Python interface. A Langevin thermostat was applied with a temperature of 298 K unless noted otherwise and a friction coefficient of 1 ps−1. As a result, the simulations described here reflect stochastic dynamics of our CG model. A value of κ = 1.5 was used to describe salt screening unless noted otherwise. The timestep for the simulations was set to 1 ps. Frames were saved every 1 ns for simulations of the 100 nm cytoplasm model, every 10 ns for the concentrated protein simulations used for parameterization, and every 100 ns for all other systems. The pairwise potential in Equation 3 was evaluated with a cutoff 49.5 nm. A switching function was applied to be effective at 49 nm. In total, about 270 ms of combined simulation time was run for all systems described here. The total computational cost for these simulations was around 350 GPU days based on timing on a single NVIDIA GeForce GTX 1080 Ti GPU card.
For validation, CG simulations of the systems with the same concentrations as in the atomistic simulations were performed for 100 μs. The CG simulations compared favorably with the atomistic simulations based on pairwise radial distribution functions and cluster size distribution (Figure 1—figure supplement 11).
Bacterial cytoplasm model
Request a detailed protocolWe constructed a coarse-grained model of Mycoplasma genitalium cytoplasm based on our previously established atomistic model (Yu et al., 2016; Feig et al., 2015). All the macromolecules and complexes were converted to single spherical particles where the particle center initially coincided with the center of mass of the molecules in the atomistic model. Sphere radii were determined as described above based on equivalent volumes, and effective charges were determined from nominal charges according to Equation 5 or Equation 6. A list of all particles with their size, charge, effective charge and concentration is given in Supplementary file 1. The initial system is a cubic box with a size of 100 nm. Additional systems were generated with 200 and 300 nm box sizes by replicating the initial system accordingly. MD simulations were run up to 1 ms as described above.
Five-component model systems
Request a detailed protocolA representative model of the cytoplasmic system consisted of five components, with an effective charge and volume fraction matching the values in the cytoplasmic system. The components consist of tRNA, ribosome particles (RP), positively charged proteins with small (POSS) and large (POSL) sizes and charges and neutral crowders (CRW) (Supplementary file 2). tRNA and RP have the same size and charge as in the full cytoplasmic system. The RP concentration includes RP, that is, complete ribosomes, in the cytoplasmic model as well as additional numbers of ribosomal fragments RR23, R50P RR16 and R30P (Supplementary file 1). The tRNA concentration was adjusted to include all particles with a nominal charge between −100 and −25, except for GroEL, which has a very large size and was not found as part of the tRNA condensates in the cytoplasmic simulations. Concentrations of the positively charged proteins were adjusted to keep the total effective charge of the system close to the cytoplasmic model. The system components were then varied to achieve different concentrations of RP and positively charged particles (Supplementary file 2). Simulations of the five-component system were performed as described above over 1 ms using only effective charges calculated via Equation 5.
Two-component model systems
Request a detailed protocolTwo-component RNA-protein systems were simulated with the same CG model as described above for 1 ms to make predictions for experimentally testable systems. Effective charges were calculated either via Equation 5 or Equation 6. RNA particles were modeled after the 47-nucleotide J345 Varkud satellite ribozyme RNA, that folds into an approximately globular shape (Bonneau and Legault, 2014) with rRNA = 1.47 nm and qRNA = −46. Proteins were considered with the following charges and radii: myoglobin (+2, 1.64 nm), trypsin (+6, 1.81 nm), lysozyme (+8, 1.54 nm), cytochrome C (+11, 1.45 nm), lactate dehydrogenase (+4, 2.68 nm), alcohol dehydrogenase (+8, 2.79 nm), and bovine serum albumin (−17, 2.58 nm).
MD simulation analysis
Analysis of the CG simulations was performed for the simulation time between 500 µs to 1 ms unless stated otherwise using in-house code in conjunction with the MMTSB Tool Set (Feig et al., 2004).
Cluster analysis
Request a detailed protocolWe previously analyzed macromolecular clustering using specific distance cutoffs that were suitable for capturing direct molecular interactions leading to transient clusters (Nawrocki et al., 2017; Nawrocki et al., 2019a; Nawrocki et al., 2019b). From those studies, we arrived at a definition of clusters based on contacts where center of mass distances between spherical particles are less than σij + 0.7 nm. σij is the pair-wise Lennard-Jones parameters in Equation 1 defined as described above in Equation 4. This criterion was applied to all pairs of particles, of same or different type, and connected graphs were generated from the pairs determined to be in contact. All particles within such a graph were then considered to be part of one cluster.
We initially applied this criterion here as well in a slightly modified version where we only considered contacts based on tRNA-protein and RP-protein pairs in order to be able to separately analyze tRNA and RP clustering in the same system. GroEL-protein pairs were also included when analyzing RP clusters since they were found to associate on the surface of the RP-rich condensates. We found that the σij + 0.7 nm contact criterion underestimated cluster sizes when visually inspecting condensed states (Figure 1—figure supplement 12). This may not be surprising since macromolecules in condensates are not necessarily in direct contact with other molecules while direct interactions are the essential feature of the transient molecular clusters described by us previously. From inspecting radial distribution functions for interactions between tRNA and POSL and POSS particles in the five-component system at different concentrations, we found that an increased cutoff of σij + 2.2 nm would include all the contacts within the first peak (Figure 2—figure supplement 9).
We further validated whether this criterion is more generally applicable to the cytoplasmic system by comparing with results from geometry-based scale-free hierarchical clustering. We applied such an algorithm to just tRNA particles during the last 100 µs of the simulation of the cytoplasmic systems so that clusters could be defined without having to invoke any contact-based criteria and without having to define clusters via interactions with other system components. We used the hierarchical clustering method implemented in the MMTSB Tool Set (Feig et al., 2004), but with a more recently established criterion for determining the optimal number of clusters (Zhou et al., 2017). This approach gave fluctuating cluster sizes between 180 and 260 tRNA molecules with a peak near 240 molecules (Figure 1—figure supplement 13). Clusters based on the σij + 2.2 nm distance cutoff for tRNA-protein pairs resulted in a narrower distribution but with a peak at the same number of molecules, whereas shorter cutoffs gave significantly smaller clusters. The broader variation in cluster sizes from the geometrical clustering reflects in part a lack of robustness in estimating optimal cluster sizes from scale-free hierarchical clustering (Zhou et al., 2017), and this is also the reason for why we used the contact-based criterion here instead of hierarchical geometrical clustering for determining tRNA and RP clusters.
Diffusion analysis
Request a detailed protocolTranslational diffusion (Dtr) was calculated for each molecule in the cytoplasmic system from the mean square displacement (MSD) of molecules between time t and (t+τ) for a given lag time τ. Diffusion coefficients were then obtained from linear fits to MSD(τ) vs. τ (Figure 1—figure supplement 6).
The first and last 1 μs of the cytoplasmic simulations were resampled so that conformations could be saved with a 1-ns interval. This allowed the analysis of all molecules in the dispersed and condensed states at the beginning and end of the trajectory and a comparison with previously published diffusion rates of macromolecules in the same system simulated in atomistic detail during similar time scales (Yu et al., 2016). In this case, the slope of MSD(τ) was fitted up until τ = 20 ns. Diffusion coefficients were calculated separately for molecules inside the tRNA and RP condensates as well as for molecules in the dilute phase. Molecules were considered to be part of a condensate if they remained part of the condensate during the entire lag time τ.
For the five-component model system, diffusion was analyzed based on the last 100 µs of the simulations based on snapshots saved with a 100-ns interval and determining the slope of MSD(τ) up until τ = 2 µs.
Phase separation analysis
Request a detailed protocolIn order to determine critical temperatures, CG simulations were performed at temperatures ranging from 300 to 500 K in 10 K increments using the Langevin thermostat. The critical temperatures and concentration were obtained by fitting the temperature to the coexisting volume fractions using the following formulas (Guggenheim, 1945):
where φH and φL are the volume fractions of tRNA inside and outside of the clusters respectively, T is the temperature, Tc is the critical temperature and φc is the critical volume fraction. This calculation was done for the model system simulations at different RP and POSL concentrations (Supplementary file 2).
Analytical theory describing condensation between RNA and proteins
Request a detailed protocolAn analytical model was constructed to reproduce the phase behavior seen in the simulations and allow a wider range of parameters to be explored. The analysis focuses on a two-component system consisting of a mixture of negatively charged particles R, equivalent to the RNA in the simulations, and particles P, equivalent to proteins, typically with a positive charge. The particles have charges qR, qP and radii rR, rP. We consider a system of volume V in which R and P particles are present in total concentrations of cR and cP. However, we do not include any finite-size effects and therefore the following analysis is scale-independent.
We assume that a phase-separated state is formed with a high-density condensate of volume Vc and a low-density dilute phase of volume Vd = V-Vc, that is, there is no change in the total system volume upon phase separation. The concentrations of R and P particles in the dilute and condensed phases are denoted as cR,d, cP,d, cR,c, and cP,c. From the concentrations, number densities , , , and for R and P particles in the dilute and condensed phases are obtained according to .
Mass conservation requires that:
and
leaving Vc and ρR,c, and ρP,c as independent variables to be determined for a given system in case of phase separation.
In general, the following scenarios are possible:
A fully disperse system, where there is no high-density condensate, that is, Vc = 0, ρR,d = ρR,, ρP,d = ρP, ρR,c = 0, and ρP,c = 0;
A fully condensed system, that is, ρR,d = 0, ρP,d = 0, ρR,c = ρR, and ρP,c = ρP;
A phase-separated system with coexistence of dilute and condensed phases for both R and P particles, that is, ρR,d > 0 and ρP,d > 0;
A phase-separated system where only R particles coexist between dilute and condensed phases, that is, ρR,d > 0, ρP,d = 0, and ρP,c = ρP;
A phase-separated system where only P particles coexist between dilute and condensed phases, that is, ρR,d = 0, ρP,d > 0, and ρR,c = ρR.
Which of these possible scenarios is assumed, depends on the total free energy of the system.
In order to determine the total free energy of the system, we begin by estimating the chemical potential for a particle either in the dilute (d) and condensed (c) phase from enthalpies and entropies according to:
In the following, only terms for the dilute phase are given. The terms for the condensed phase are obtained in an equivalent manner.
The enthalpy terms are decomposed into interactions of R-R, P-P, and R-P pairs:
Each pairwise interaction energy is estimated from the coarse-grained interaction potential by assuming a spherically symmetric distribution of particles but modulated as a function of distance according to radial distribution function extracted from simulations for each pair. This amounts to convoluting the pairwise interaction potential U (see Equation 3) with scaled volume- and density-normalized radial distribution functions as follows:
where the factor 1/2 corrects for double-counted self-interactions.
Different radial distribution functions were used for dilute and condensed environments (Figure 2—figure supplement 10). The g(r) functions extracted from the simulations were truncated at 20 nm and set to a constant value of 1 for larger radii to remove finite-size artifacts. Although the g(r) functions were determined from simulations with specific sizes rR,MD, rP,MD of the R and P particles, other particle sizes could be considered by scaling the radial dependence of the g(r) functions according to the ratios rR/rR,MD, rP/rP,MD, and (rR+rP)/(rR,MD+rP,MD) for R-R, P-P, and R-P interactions. The upper integration limit rmax was set to 100 nm for all interactions. At that radius and above, U(r) is negligible for the range of radii and charges considered here. With the fixed integration limit, the integrals in Equations 15 to 18 vary only with the charges and radii of particles R and P, and, thus, they are independent of particle concentrations. Then, the enthalpy contributions can be written as:
where the x values represent the integrals in Equations 15 to 18 multiplied by 2π.
The entropy term was calculated based on the change of concentration in either dilute or condensed phases relative to the concentration in a fully disperse, non-separated system, which is the total system concentration, that is, for the dilute phase:
where R is the universal gas constant. In estimating the entropy for the condensed phase, the finite volumes of the R and P particles were subtracted from the condensed phase volume Vc:
with the molecular volumes calculated from the radii of the spherical R and P particles:
and
Coexistence of the dilute and condensed phases assumes equilibrium, that is:
In scenario (3), both, Equations 28 and 29, have to be satisfied simultaneously. For scenario (4), only Equation 28 needs to be satisfied under the condition that ρP,d = 0; and for scenario (5), only Equation 29 has to be satisfied with ρR,d = 0.
Solutions in terms of ρR,d, ρP,d, ρR,c, ρP,c, and Vc were determined numerically by scanning Vc and solving for the densities in the dilute phase (the densities in the condensed phase follow from Equation 11).
Equation 28 combined with Equations 11, 12, 13, 19, 21, 23, and 25 gives the following:
An analogous function is obtained from Equation 29. There is no analytical solution, but and can be solved via the Newton-Raphson method given Vc and either ρP,d or ρR,d.
For scenario (4), was solved for different values of Vc and ρP,d = 0; for scenario (5), was solved for values of Vc and ρR,d = 0. For scenario (3), ρR,d was scanned as well and the value of ρP,d was determined for given values of Vc and ρR,d by first solving . The resulting value of ρP,d was then used with Vc to solve for a refined value of ρR,d.
Mathematically possible solutions include cases where the volume fractions in the cluster exceed what is physically realistic inside the condensed state. In order to exclude such solutions, it was required that the combined macromolecular volume in the condensed phase is less than 30% of the total volume of the condensed phase, that is,:
We note that most final solutions were found at the 30% vol fraction limit, since the theory did not directly account for volume exclusion between individual molecules and found a gain in energy at higher particle densities. However, similar results were obtained with maximal macromolecular volume fractions according to Equation 32 in a range of 20–40%. The value of 30% was ultimately arrived at by optimal agreement between theory and experiment for the concentration-dependent phase separation between RNA and trypsin shown in Figure 6.
The total system energy is calculated according to:
where Smix is the overall mixing entropy according to the ratio of particles R and P in the dilute and condensed phases according to:
For the five scenarios described above, total free energies were then calculated as follows:
(1) Disperse:
where were calculated according to Equations 12 to 18 using RDFs from the disperse phase extracted from our molecular dynamics simulations before condensates started to form.
(2) Condensed:
(3) R and P in phase coexistence:
since µR,c = µR,d and µP,c = µP,d.
(4) R in phase coexistence, ρP,d = 0:
(5) P in phase coexistence, ρR,d = 0:
The scenario with the overall lowest free energy was then considered to be the predicted state.
A program implementing this model is available at http://github.com/feiglab/phasesep; copy archived at swh:1:rev:24890516a822b917b76a2730ced19839acbaec3d
Experimental materials and methods
The J345 RNA sequence was synthesized and deprotected by Dharmacon (Horizon Discovery Group), both with and without Cy3 or Cy5 on the 3’ end. The 47-base sequence is GCAGCAGGGAACUCACGCUUGCGUAGAGGCUAAGUGCUUCGGCACAGCACAAGCCCGCUGCG.
All measurements were made using the buffer used by Bonneau and Legault for structure determination of this sequence, 10 mM sodium cacodylate (pH 6.5), 50 mM NaCl,. 05% sodium azide, 5 mM MgCl2. Equine liver trypsin, equine alcohol dehydrogenase, bovine lactic dehydrogenase, equine myoglobin, hen egg lysozyme, and bovine serum albumin were obtained from Sigma-Aldrich and used without further modification.
Microscopy
Request a detailed protocolConfocal microscopy images were obtained on a Nikon A1 scanning confocal microscope with 100x magnification. The excitation wavelength was 561 nm and detection was set for Cy3 fluorescence using a GaAsP detector. The diffraction-limited spatial resolution is 260 nm. Images were processed with ImageJ and modified only for contrast and brightness. Images were cropped and enlarged to aid observation of the smallest features.
Dynamic light scattering
Request a detailed protocolThe size distribution of the protein-RNA complexes were measured using a dynamic light scattering (DLS) machine (Zetasizer nano series from Malvern company) at room temperature. The samples were mixed freshly before each experiment and all measurements were repeated three times in a single run and the corresponding average results were reported. A Helium Neon laser with a wavelength of 632 nm was used for the size distribution analysis.
The central observable of dynamic light scattering (DLS) experiments consists of time-dependent scattering intensity correlation functions g2(τ) that are related to electric field correlation functions g1(τ) according to:
In case of a monodisperse solution with particles of a diameter d, a single exponential decay is observed with:
with the wave vector
and the diffusion according to Stokes-Einstein:
where n is the refractive index of the solvent medium (i.e. 1.335), λ is the wavelength of the incident laser light (i.e. 633 nm), θ is the scattering angle (i.e. 173°), kB is the Boltzmann constant, T is the temperature (i.e. 298 K), and η is the viscosity of the solvent (i.e. 0.8882 cP).
The samples we considered were clearly polydisperse, requiring the fit of multiple exponential decays. Moreover, from previous studies and simulations, we expect that at the smallest particle sizes there is an exponential decay of particle sizes due to dynamic cluster formation in the dilute phase (Nawrocki et al., 2019b, von Bülow et al., 2019). Therefore, we fit the experimental data (i.e. ) to the following function:
Consequently, the parameters of the numerical fits were the size of the smallest particle, dc, its contribution, ac, decreasing according to the decay ‘time’ tc, and an additional up to four discrete sizes di with contributions ai.
Using gnuplot, version 5.2, we fit the function according to Equation 46 to individual correlation functions as well to an average that was obtained after normalizing individual functions.
FRET spectroscopy
Request a detailed protocolFluorescence spectra were obtained with PTI Q4 fluorimeter, excited at 475 nm and emission observed between 525 and 700 nm. The concentration of Cy3-labeled RNA and Cy5-labeled RNA were kept constant at 8 µM and 42 µM, respectively, with the unlabeled concentration varied from 0 to 0.5 mM. The low concentration of labeled RNA limits the possibility of self-quenching but also limits the detection of very small clusters.
The normalized FRET ratio was calculated from the total intensity between 525 and 650 nm for the donor and 650 and 700 nm for the acceptor,
In the absence of protein, the RNA exhibits some baseline transfer, likely due to transient interactions between the dyes, leading to a background FRET level of ~0.24. Upon the addition of protein above the threshold concentration, the mixture is visibly turbid.
FRET efficiencies for mixtures of RNA and proteins at different concentrations were estimated from the predicted amount of RNA inside and outside the condensates as follows:
The theory described above predicts phase separation with the densities of RNA in the dilute and condensed phases given as ρR,d and ρR,c. From the densities the concentration of RNA in the dilute ([Rd]) and condensed ([Rc]) phases with respect to the total volume is obtained as follows:
A fraction of RNA is labeled with fluorophores. The total concentration of labeled RNA is denoted as [F]; the concentration in the dilute and condensed phases, again with respect to the total system volume, is denoted as [Fd] and [Fc], respectively. Then:
and
where [Ud] and [Uc] are the concentrations of unlabeled RNA in the dilute and condensed phases.
We further make an assumption that there is an equilibrium of labeled RNA to exchange between the dilute and condensed phases while maintaining the overall ratio of RNA between the two phases:
with the equilibrium constant K given as:
Because of the hydrophobic character of the FRET labels we expect that labeled RNA has an affinity for the less-hydrated condensate, that is, K > 1.
Equations 50, 51, 52, 54 can be solved for [Fc] as a function of [Rd], [Rc], [F], and K to give the fraction of labeled RNA in the condensate as:
Based on the resulting value of f, FRET efficiencies E were then estimated according to:
where E0 and Ec are the FRET efficiencies at zero protein concentration and in the condensed phase, respectively. E0 was taken from experiment and Ec was estimated by convoluting the distribution of minimum RNA-RNA distances in the condensed phase extracted from the simulations with 1/ (1+(r/r0)6), where r is the distance between RNA molecules and r0 is a constant that depends on the fluorescence label and additional factors such as the anisotropy of the orientational sampling and the index of diffraction of the medium.
We applied this formalism to interpret the FRET experiments on trypsin based on predicted RNA fractions in the condensed phase (Figure 9—figure supplement 7) using the minimum distance distribution of RNA shown in Figure 2—figure supplement 11. We took E0 = 0.24 from experiment and found good agreement between experiment and theory for r0 = 4.10 nm and K = 100 (Figure 7). We note that the value r0 = 4.10 nm is lower than typical values assumed for the Cy3-Cy5 pair (Murphy et al., 2004), but the condensed state differs from typical solution conditions, whereas the spherical models used here allow only very approximate estimates of the true donor-acceptor distances and neglect orientational dependence in fluorescent energy transfer (Iqbal et al., 2008).
NMR spectroscopy
Request a detailed protocolNMR spectra were acquired at a 1H frequency of 600 MHz on a Varian 600 MHz spectrometer with a room-temperature probe. Solvent was suppressed with a gradient 1–1 echo sequence. Samples were prepared in 90% H2O, 10% D2O in the buffer described above with DSS as an internal chemical shift reference. 16 k points were acquired with a 1 s recycle delay and a total acquisition time of approximately 1 hr per spectrum. RNA concentrations were 300 µM for J345 only and 135–140 µM for RNA-protein samples; protein concentrations were around 150 µM; the RNA-only spectrum was scaled to account for the differing concentration. Spectra were processed with zero-filling to 32 k and a 5 Hz exponential window function.
Circular dichroism spectroscopy
Request a detailed protocolCircular dichroism measurements were made using an Applied Photophysics Chirascan spectrometer. All measurements were made using a 0.1 mm pathlength cuvette at room temperature.
Data availability
All experimental data generated and analyzed during this study are included in the manuscript and supporting files.
References
-
Defect in the formation of 70S ribosomes caused by lack of ribosomal protein L34 can be suppressed by magnesiumJournal of Bacteriology 196:3820–3830.https://doi.org/10.1128/JB.01896-14
-
Interaction between particles suspended in solutions of macromoleculesJournal of Polymer Science 33:183–192.https://doi.org/10.1002/pol.1958.1203312618
-
Biomolecular condensates: organizers of cellular biochemistryNature Reviews Molecular Cell Biology 18:285–298.https://doi.org/10.1038/nrm.2017.7
-
NMR detection of an equilibrium phase consisting of monomers and clusters in concentrated lysozyme solutionsThe Journal of Physical Chemistry B 114:17062–17067.https://doi.org/10.1021/jp108995k
-
Stochastic dynamics simulations of macromolecular diffusion in a model of the cytoplasm of Escherichia coliThe Journal of Physical Chemistry 100:2489–2497.https://doi.org/10.1021/jp9525191
-
Protein phase separation: a new phase in cell biologyTrends in Cell Biology 28:420–435.https://doi.org/10.1016/j.tcb.2018.02.004
-
The multifunctional nucleolusNature Reviews Molecular Cell Biology 8:574–585.https://doi.org/10.1038/nrm2184
-
Polymer physics of intracellular phase transitionsNature Physics 11:899–904.https://doi.org/10.1038/nphys3532
-
Architectural RNAs (arcRNAs): A class of long noncoding RNAs that function as the scaffold of nuclear bodiesBiochimica Et Biophysica Acta (BBA) - Gene Regulatory Mechanisms 1859:139–146.https://doi.org/10.1016/j.bbagrm.2015.05.007
-
Cajal bodies: a long history of discoveryAnnual Review of Cell and Developmental Biology 21:105–131.https://doi.org/10.1146/annurev.cellbio.20.010403.103738
-
Complex coacervation of proteins and anionic polysaccharidesCurrent Opinion in Colloid & Interface Science 9:340–349.https://doi.org/10.1016/j.cocis.2004.09.006
-
Effective charge of colloidal particlesThe Journal of Chemical Physics 121:12100–12103.https://doi.org/10.1063/1.1824013
-
Sequence determinants of protein phase behavior from a coarse-grained modelPLOS Computational Biology 14:e1005941.https://doi.org/10.1371/journal.pcbi.1005941
-
Simulation methods for liquid-liquid phase separation of disordered proteinsCurrent Opinion in Chemical Engineering 23:92–98.https://doi.org/10.1016/j.coche.2019.03.004
-
Who's in and who's Out-Compositional control of biomolecular condensatesJournal of Molecular Biology 430:4666–4684.https://doi.org/10.1016/j.jmb.2018.08.003
-
OpenMM 7: rapid development of high performance algorithms for molecular dynamicsPLOS Computational Biology 13:e1005659.https://doi.org/10.1371/journal.pcbi.1005659
-
Nucleation by rRNA dictates the precision of nucleolus assemblyCurrent Biology 26:277–285.https://doi.org/10.1016/j.cub.2015.11.065
-
The role of RNA in biological phase separationsJournal of Molecular Biology 430:4685–4701.https://doi.org/10.1016/j.jmb.2018.05.003
-
Quantitative analysis of multilayer organization of proteins and RNA in nuclear speckles at super resolutionJournal of Cell Science 130:4180–4192.https://doi.org/10.1242/jcs.206854
-
MMTSB tool set: enhanced sampling and multiscale modeling methods for applications in structural biologyJournal of Molecular Graphics and Modelling 22:377–395.https://doi.org/10.1016/j.jmgm.2003.12.005
-
Complete atomistic model of a bacterial cytoplasm for integrating physics, biochemistry, and systems biologyJournal of Molecular Graphics and Modelling 58:1–9.https://doi.org/10.1016/j.jmgm.2015.02.004
-
Crowding in cellular environments at an atomistic level from computer simulationsThe Journal of Physical Chemistry B 121:8009–8025.https://doi.org/10.1021/acs.jpcb.7b03570
-
Whole-Cell models and simulations in molecular detailAnnual Review of Cell and Developmental Biology 35:191–211.https://doi.org/10.1146/annurev-cellbio-100617-062542
-
In vitro reconstitution of a cellular phase-transition process that involves the mRNA decapping machineryAngewandte Chemie International Edition 53:7354–7359.https://doi.org/10.1002/anie.201402885
-
Nuclear speckles: molecular organization, biological function and role in diseaseNucleic Acids Research 45:10350–10368.https://doi.org/10.1093/nar/gkx759
-
Cajal bodies: the first 100 yearsAnnual Review of Cell and Developmental Biology 16:273–300.https://doi.org/10.1146/annurev.cellbio.16.1.273
-
The principle of corresponding statesThe Journal of Chemical Physics 13:253–261.https://doi.org/10.1063/1.1724033
-
Nucleolus: a central hub for nuclear functionsTrends in Cell Biology 29:647–659.https://doi.org/10.1016/j.tcb.2019.04.003
-
Equilibrium clusters in concentrated lysozyme protein solutionsJournal of Colloid and Interface Science 363:579–584.https://doi.org/10.1016/j.jcis.2011.07.043
-
Nuclear speckles: a model for nuclear organellesNature Reviews Molecular Cell Biology 4:605–612.https://doi.org/10.1038/nrm1172
-
Sequence-Specific polyampholyte phase separation in membraneless organellesPhysical Review Letters 117:178101.https://doi.org/10.1103/PhysRevLett.117.178101
-
Multiphase complex coacervate dropletsJournal of the American Chemical Society 142:2905–2914.https://doi.org/10.1021/jacs.9b11468
-
P-Bodies: composition, properties, and functionsBiochemistry 57:2424–2431.https://doi.org/10.1021/acs.biochem.7b01162
-
Cajal bodies: where form meets functionWiley Interdisciplinary Reviews: RNA 4:17–34.https://doi.org/10.1002/wrna.1139
-
The molecular theory of polyelectrolyte solutions with applications to the electrostatic properties of polynucleotidesQuarterly Reviews of Biophysics 11:179–246.https://doi.org/10.1017/S0033583500002031
-
Protein-Polyelectrolyte phase boundariesBiotechnology Progress 11:632–637.https://doi.org/10.1021/bp00036a005
-
Diffusion, crowding & protein stability in a dynamic molecular model of the bacterial cytoplasmPLOS Computational Biology 6:e1000694.https://doi.org/10.1371/journal.pcbi.1000694
-
Phase separation of polyelectrolyte solutionsJournal of Polymer Science 23:443–450.https://doi.org/10.1002/pol.1957.1202310337
-
Methods for physical characterization of Phase-Separated bodies and Membrane-less organellesJournal of Molecular Biology 430:4773–4805.https://doi.org/10.1016/j.jmb.2018.07.006
-
Slow-Down in diffusion in crowded protein solutions correlates with transient cluster formationThe Journal of Physical Chemistry B 121:11072–11084.https://doi.org/10.1021/acs.jpcb.7b08785
-
Effect of protein-protein interactions and solvent viscosity on the rotational diffusion of proteins in crowded environmentsPhysical Chemistry Chemical Physics 21:876–883.https://doi.org/10.1039/C8CP06142D
-
Phase separation in polyelectrolyte solutions; theory of complex coacervationJournal of Cellular and Comparative Physiology 49:7–26.https://doi.org/10.1002/jcp.1030490404
-
Phase separation of intrinsically disordered proteinsMethods in Enzymology 611:1–30.https://doi.org/10.1016/bs.mie.2018.09.035
-
Principles and properties of stress granulesTrends in Cell Biology 26:668–679.https://doi.org/10.1016/j.tcb.2016.05.004
-
Protein folding, binding, and droplet formation in cell-like conditionsCurrent Opinion in Structural Biology 43:28–37.https://doi.org/10.1016/j.sbi.2016.10.006
-
Sequence dependent phase separation of protein-polynucleotide mixtures elucidated using molecular simulationsNucleic Acids Research 48:12593–12603.https://doi.org/10.1093/nar/gkaa1099
-
Advances in understanding Stimulus-Responsive phase behavior of intrinsically disordered protein polymersJournal of Molecular Biology 430:4619–4635.https://doi.org/10.1016/j.jmb.2018.06.031
-
Membraneless nuclear organelles and the search for phases within phasesWiley Interdisciplinary Reviews: RNA 10:e1514.https://doi.org/10.1002/wrna.1514
-
Development of the modern theory of polymeric complex coacervationAdvances in Colloid and Interface Science 239:2–16.https://doi.org/10.1016/j.cis.2016.04.004
-
Binodal compositions of polyelectrolyte complexesMacromolecules 43:6476–6484.https://doi.org/10.1021/ma101031t
-
Why does RNA collapse? the importance of water in a simulation study of Helix-Junction-Helix systemsJournal of the American Chemical Society 140:16948–16951.https://doi.org/10.1021/jacs.8b11111
-
Diffusion within the cytoplasm: a mesoscale model of interacting macromoleculesBiophysical Journal 107:2579–2591.https://doi.org/10.1016/j.bpj.2014.09.043
-
Ribosome motions modulate electrostatic propertiesBiopolymers 74:423–431.https://doi.org/10.1002/bip.20093
-
RNA granules in germ cellsCold Spring Harbor Perspectives in Biology 3:a002774.https://doi.org/10.1101/cshperspect.a002774
-
A physics-based approach of coarse-graining the cytoplasm of Escherichia coli (CGCYTO)Biophysical Journal 102:2353–2361.https://doi.org/10.1016/j.bpj.2012.04.010
-
Sequence-encoded material properties dictate the structure and function of nuclear bodiesCurrent Opinion in Cell Biology 46:62–71.https://doi.org/10.1016/j.ceb.2017.03.003
-
Predicting protein interactions of concentrated globular protein solutions using colloidal modelsThe Journal of Physical Chemistry B 121:4756–4767.https://doi.org/10.1021/acs.jpcb.7b02183
-
Competitive binding of cations to duplex DNA revealed through molecular dynamics simulationsThe Journal of Physical Chemistry B 116:12946–12954.https://doi.org/10.1021/jp306598y
-
Method for determining the optimal number of clusters based on agglomerative hierarchical clusteringIEEE Transactions on Neural Networks and Learning Systems 28:3007–3017.https://doi.org/10.1109/TNNLS.2016.2608001
-
Macromolecular crowding: biochemical, biophysical, and physiological consequencesAnnual Review of Biophysics and Biomolecular Structure 22:27–65.https://doi.org/10.1146/annurev.bb.22.060193.000331
Article and author information
Author details
Funding
National Institutes of Health (R35 GM126948)
- Bercem Dutagaci
- Grzegorz Nawrocki
- Michael Feig
National Science Foundation (MCB 1817307)
- Lisa J Lapidus
- Michael Feig
National Science Foundation (MCB 2018296)
- Charles G Hoogstraten
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
This study was funded by the National Science Foundation (MCB 1817307, to MF and LJL; MCB 2018296, to CGH) and the National Institutes of Health (R35 GM126948, to MF). Computational resources at the Institute for Cyber-Enabled Research/High Performance Computing Cluster (ICER/HPCC) at Michigan State University were used.
Version history
- Received: October 15, 2020
- Accepted: January 25, 2021
- Accepted Manuscript published: January 26, 2021 (version 1)
- Version of Record published: February 10, 2021 (version 2)
Copyright
© 2021, Dutagaci et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 6,299
- views
-
- 728
- downloads
-
- 39
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Physics of Living Systems
Eukaryotes swim with coordinated flagellar (ciliary) beating and steer by fine-tuning the coordination. The model organism for studying flagellate motility, Chlamydomonas reinhardtii, employs synchronous, breaststroke-like flagellar beating to swim, and it modulates the beating amplitudes differentially to steer. This strategy hinges on both inherent flagellar asymmetries (e.g. different response to chemical messengers) and such asymmetries being effectively coordinated in the synchronous beating. In C. reinhardtii, the synchrony of beating is known to be supported by a mechanical connection between flagella; however, how flagellar asymmetries persist in the synchrony remains elusive. For example, it has been speculated for decades that one flagellum leads the beating, as its dynamic properties (i.e. frequency, waveform, etc.) appear to be copied by the other one. In this study, we combine experiments, computations, and modeling efforts to elucidate the roles played by each flagellum in synchronous beating. With a non-invasive technique to selectively load each flagellum, we show that the coordinated beating essentially only responds to load exerted on the cis flagellum; and that such asymmetry in response derives from a unilateral coupling between the two flagella. Our results highlight a distinct role for each flagellum in coordination and have implication for biflagellates’ tactic behaviors.
-
- Developmental Biology
- Physics of Living Systems
Geometric criteria can be used to assess whether cell intercalation is active or passive during the convergent extension of tissue.






