Cancer Immunotherapy: A promising platform for predicting toxicity
Killing cancer cells can be done directly or by harnessing the immune system through an approach called cancer immunotherapy (Robert, 2020). Indeed, over the past decade, most of the advances in oncology have involved boosting immune cells to destroy tumor cells. Some of these treatments have resulted in impressive improvements in survival, bringing the possibility of a cure closer for some patients (Robert et al., 2018). This is a big milestone in oncology.
Despite these advances, immunotherapies can be associated with toxicity, which forces the treatment to be stopped: if immune cells become too activated, they can mistakenly recognize and destroy healthy tissues. This can lead, for example, to rashes, hepatitis or colitis depending on whether the skin, liver or gut are attacked (Marin-Acevedo et al., 2019).
A big challenge in oncology is therefore to predict which new immunotherapy drugs are going to be too harmful for patients. This is usually examined in animal models, but since their immune systems differ from the human immune system, it can be difficult to reliably predict toxicity (Zschaler et al., 2014). Now, in eLife, Nikolce Gjorevski (Roche), Lauriane Cabon (Roche) and colleagues – including Jordan Kerns and Chaitra Belgur of Emulate Inc in Boston as joint first authors – report how an in vitro model can help bypass this problem for T cell bispecific antibodies immunotherapy (Kerns et al., 2021).
T cell bispecific antibodies (or TCBs) can recognise and bind to ‘antigen’ proteins present on the surface of tumors, as well as receptors displayed by immune ‘T cells’: by bringing the two types of cells closer, this process helps to activate T cells and allows them to kill their targets. However, the antigens that TCBs bind to are not always exclusive to cancer cells. Recognition of non-cancer cells which share antigens with tumors – known as the on-target, off-tumor effect – can lead to normal cells being damaged (Labrijn et al., 2019; Figure 1). Predicting which TCBs under clinical development will cause such undesired toxicity represents an important challenge in oncology.
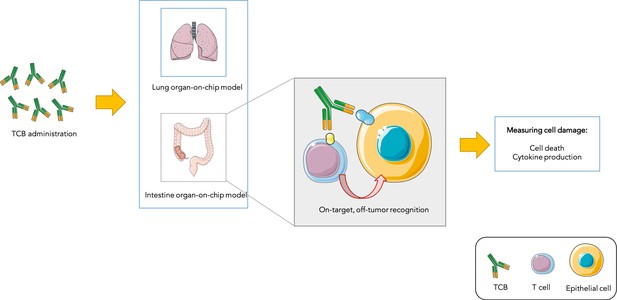
Testing drug toxicity using an organ-on-chip platform.
Administering TCBs to an organ-on-chip platform (such as a model of the lung or intestine) allows the antibodies (Y-shaped structures) to recognize antigens (shown in yellow and blue) present in both healthy epithelial and cancerous cells. This brings T cells close to their targets and also activates them, which can lead to healthy cells being damaged (as assessed by measuring cell death and inflammation levels). This model is able to partially reproduce toxicity observed in animals and humans.
Image credit: Image was prepared with Servier Medical Art (CC BY 3.0).
To address this issue, Kerns et al. first took advantage of a lung-on-chip model (Huh et al., 2010) – a system grown under conditions mimicking those found in the body – to predict toxicity to TCBs. This ‘mini-organ’ was exposed to a TCB that recognises an antigen present in ovarian, lung and breast cancer cells, but which can also be expressed, at lower levels, in healthy lung cells. This manipulation led to healthy cells being damaged in the lung-on-chip model, which was then used to determine which TCB dose could kill tumors while sparing normal lung cells. This dose was then administered to mouse models, whose lungs remained undamaged. This demonstrates that the lung-on-chip was able to efficiently predict toxicity in these animals.
Kerns et al. then used an intestine-on-chip model to test a TCB which targets an antigen present on both colon cancer and normal intestine cells. Healthy cells survived the treatment but signs of toxicity emerged that could mimick side-effects that commonly occur in patients, such as increased inflammation.
Using an organ-on-chip model to test TCBs therefore has two main advantages. First, it reproduces toxicity observed in vivo, and can help to determine which doses are effective while remaining safe for normal cells. Second, it serves to predict toxicity before patients are administered with newly-developed TCBs, filling the gap between animal and human studies for drugs that are yet to be clinically tested. Organ-on-chip models could therefore make drug development more efficient by helping to screen out toxic TCBs before they reach cancer patients.
References
-
Bispecific antibodies: a mechanistic review of the pipelineNature Reviews Drug Discovery 18:585–608.https://doi.org/10.1038/s41573-019-0028-1
-
Immune checkpoint inhibitor toxicitiesMayo Clinic Proceedings 94:1321–1329.https://doi.org/10.1016/j.mayocp.2019.03.012
-
Durable complete response after discontinuation of pembrolizumab in patients with metastatic melanomaJournal of Clinical Oncology 36:1668–1674.https://doi.org/10.1200/JCO.2017.75.6270
-
A decade of immune-checkpoint inhibitors in cancer therapyNature Communications 11:3801.https://doi.org/10.1038/s41467-020-17670-y
-
Differences in innate immune response between man and mouseCritical Reviews in Immunology 34:433–454.https://doi.org/10.1615/CritRevImmunol.2014011600
Article and author information
Author details
Publication history
- Version of Record published: September 24, 2021 (version 1)
Copyright
© 2021, Ochoa de Olza
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 856
- views
-
- 63
- downloads
-
- 2
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Cancer Biology
- Cell Biology
Pancreatic ductal adenocarcinoma carries a dismal prognosis, with high rates of metastasis and few treatment options. Hyperactivation of KRAS in almost all tumours drives RAC1 activation, conferring enhanced migratory and proliferative capacity as well as macropinocytosis. Macropinocytosis is well understood as a nutrient scavenging mechanism, but little is known about its functions in trafficking of signaling receptors. We find that CYRI-B is highly expressed in pancreatic tumours in a mouse model of KRAS and p53-driven pancreatic cancer. Deletion of Cyrib (the gene encoding CYRI-B protein) accelerates tumourigenesis, leading to enhanced ERK and JNK-induced proliferation in precancerous lesions, indicating a potential role as a buffer of RAC1 hyperactivation in early stages. However, as disease progresses, loss of CYRI-B inhibits metastasis. CYRI-B depleted tumour cells show reduced chemotactic responses to lysophosphatidic acid, a major driver of tumour spread, due to impaired macropinocytic uptake of the lysophosphatidic acid receptor-1. Overall, we implicate CYRI-B as a mediator of growth and signaling in pancreatic cancer, providing new insights into pathways controlling metastasis.
-
- Cancer Biology
- Cell Biology
Internalization from the cell membrane and endosomal trafficking of receptor tyrosine kinases (RTKs) are important regulators of signaling in normal cells that can frequently be disrupted in cancer. The adrenal tumor pheochromocytoma (PCC) can be caused by activating mutations of the rearranged during transfection (RET) receptor tyrosine kinase, or inactivation of TMEM127, a transmembrane tumor suppressor implicated in trafficking of endosomal cargos. However, the role of aberrant receptor trafficking in PCC is not well understood. Here, we show that loss of TMEM127 causes wildtype RET protein accumulation on the cell surface, where increased receptor density facilitates constitutive ligand-independent activity and downstream signaling, driving cell proliferation. Loss of TMEM127 altered normal cell membrane organization and recruitment and stabilization of membrane protein complexes, impaired assembly, and maturation of clathrin-coated pits, and reduced internalization and degradation of cell surface RET. In addition to RTKs, TMEM127 depletion also promoted surface accumulation of several other transmembrane proteins, suggesting it may cause global defects in surface protein activity and function. Together, our data identify TMEM127 as an important determinant of membrane organization including membrane protein diffusability and protein complex assembly and provide a novel paradigm for oncogenesis in PCC where altered membrane dynamics promotes cell surface accumulation and constitutive activity of growth factor receptors to drive aberrant signaling and promote transformation.






