A systematic assessment of preclinical multilaboratory studies and a comparison to single laboratory studies
Figures
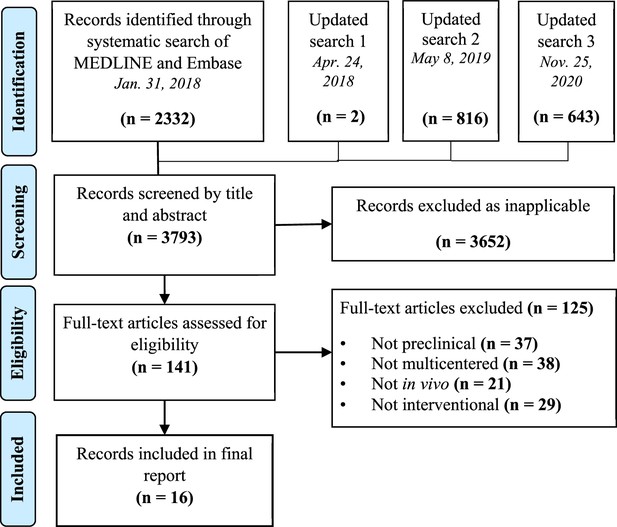
Preferred reporting items for systematic reviews and meta-analysis (PRISMA) flow diagram for study selection.
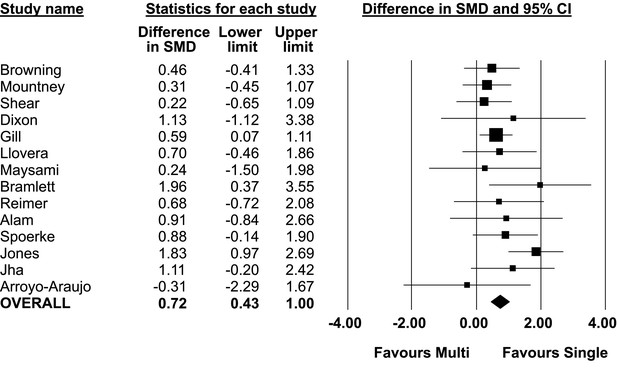
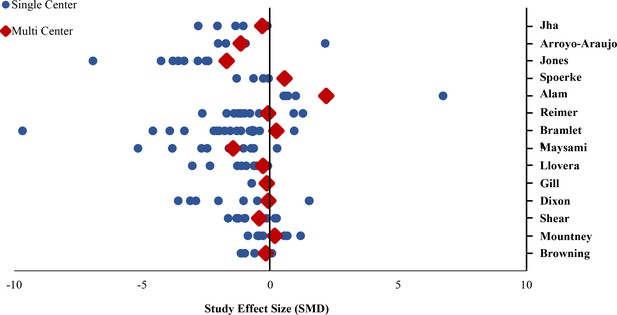
Difference in standardized mean difference between single lab and multilaboratory preclinical studies.
-
Figure 2—source data 1
Source data for comparision of single lab and multilaboratory studies.
- https://cdn.elifesciences.org/articles/76300/elife-76300-fig2-data1-v2.xlsx
-
Figure 2—source data 2
Standardized mean differences for all 14 singlevs multilaboratory comparisons and sub-groups by total quality score.
- https://cdn.elifesciences.org/articles/76300/elife-76300-fig2-data2-v2.pdf
Tables
Basic study characteristics of preclinical multilaboratory studies.
| Author, Year | Center location | Journal | Funding | Centers PerformingIn Vivo Work | Non-Experimental Centers* | Animal, Sex | Sample size |
|---|---|---|---|---|---|---|---|
| Reimer et al., 1985 | US | Circulation Research | Government (NHLBI) | 3 | 1 | Dog, both | 51 |
| Crabbe et al., 1999 | Canada, US | Science | Government | 3 | 0 | Mouse, both | 384 |
| Alam et al., 2009 | US | Journal of Trauma: Injury, Infection and Critical Care | Government (US army) | 3 | 0 | Swine, F | 60 |
| Spoerke et al., 2009 | US | Archives of Surgery | Government (US army) | 2 | 0 | Swine, NA | 32 |
| Jones et al., 2015 | US | Circulation Research | Government (NHLBI) | 3 | 3 | Mouse, M Rabbit, M Swine, F | 47 23 26 |
| Llovera et al., 2015 | France, Germany, Italy, Spain | Science Translational Medicine | Government, academic and charitable | 5 | 1 | Mouse, M | 315 |
| Maysami et al., 2016 | Finland, France, Germany, Hungary, UK, Spain | Journal of Cerebral Blood Flow & Metabolism | Government (FP7/ 2007–2013, INSERM), academic | 5 | 1† | Mouse, M | 241 |
| Bramlett et al., 2016 | US | Journal of Neurotrauma | Government (US army) | 3 | 1 | Rat, M | 140 |
| Browning et al., 2016 | US | Journal of Neurotrauma | Government (US army) | 3 | 1 | Rat, M | 130 |
| Dixon et al., 2016 | US | Journal of Neurotrauma | Government (US army) | 3 | 1 | Rat, M | 135 |
| Gill et al., 2016 | US | Diabetes | Government, charitable | 4 | 0 | Mouse, F | NR |
| Mountney et al., 2016 | US | Journal of Neurotrauma | Government (US army) | 3 | 1 | Rat, M | 128 |
| Shear et al., 2016 | US | Journal of Neurotrauma | Government (US army) | 3 | 1 | Rat, M | 142 |
| Arroyo-Araujo et al., 2022 | Netherlands, Switzerland, US | Scientific Reports | Government (FP7/2007–2013), industry (EFPIA), charitable | 3 | 0 | Rat, M | 72 |
| Jha et al., 2020 | US | Journal of Neurotrauma | Government (US army) | 3 | 1 | Rat, M | 111 |
| Kliewer et al., 2020 | Australia, Germany, UK | British Journal of Pharmacology | Government (NHMRC, NIH), NGO | 3 | 0 | Mouse, M | 108 |
-
Legend: EFPIA - European Federation of Pharmaceutical Industries and Associations; FP7/2007-2013 – European Union Commission seventh Funding Program; INSERM - Institut national de la santé et de la recherche médicale; NGO – Non-government organization; NHLBI – National Heart, Lung, and Blood Institute; NHMRC – National Health and Medical Research Council; NIH – National Institutes of Health; NR - not reported; UK – United Kingdom; US – United States.
-
*
Non-experimental center: A site/lab not involved with the in vivo experiment (data processing, coordinating, biomarker, or pathology centers).
-
†
Center that was both an experimental center and a coordinating center.
Study design characteristics of preclinical multilaboratory studies.
| Author, Year | Disease model | Intervention | Study Outcomes | Secondary Outcomes | Reported Results | Recommendations for Future Research |
|---|---|---|---|---|---|---|
| Reimer et al., 1985 | Myocardial infarction | Verapamil and ibuprofen | Infarct size | Mortality, hemodynamic measures, pathological/histological features, regional blood flow | Null | Not reported |
| Crabbe et al., 1999 | Stimulant exposure | Cocaine | Locomotor activity | Mixed | Further preclinical testing | |
| Alam et al., 2009 | Polytrauma | Blood transfusion | Hemodynamic parameters | Mortality | Mixed across resuscitation products | Further preclinical testing |
| Spoerke et al., 2009 | Polytrauma | Lyophilized plasma | Residual clotting activity | Mortality, hemodynamic measures, total blood loss, coagulation profiles, inflammatory measures | Positive | Further preclinical testing |
| Jones et al., 2015 | Myocardial infarction | Ischemic preconditioning | Infarct size | Hemodynamic measures, regional blood flow, heart weight, troponin I, mean arterial pressure | Positive | Further preclinical testing |
| Llovera et al., 2015 | Stroke | Anti-CD49d antibody | Infarct size | Functional outcome, invasion of leukocytes to brain | Mixed across models (positive, null) | First-in-human clinical trial |
| Maysami et al., 2016 | Stroke | Interleukin-I receptor antagonist | Infarct size | Odema, functional outcome, mortality | Positive | Extensive clinical trial |
| Bramlett et al., 2016 | Traumatic brain injury | Erythropoietin | Cognitive outcomes, biomarkers, motor outcomes, neuropathology | Null | No further preclinical study | |
| Browning et al., 2016 | Traumatic brain injury | Levetiracetam | Cognitive outcomes, biomarkers, motor outcomes, neuropathology | Positive | Further preclinical testing and first-in-human clinical trial | |
| Dixon et al., 2016 | Traumatic brain injury | Cyclosporine | Cognitive outcomes, biomarkers, motor outcomes, neuropathology | Null | No further preclinical testing | |
| Gill et al., 2016 | Diabetes | Combined anti-CD3 +IL-1 blockade | Blood glucose | Null | Pause clinical trial | |
| Mountney et al., 2016 | Traumatic brain injury | Simvastatin | Cognitive outcomes, biomarkers, motor outcomes, neuropathology | Null | No further preclinical study | |
| Shear et al., 2016 | Traumatic brain injury | Nicotinamide | Cognitive outcomes, biomarkers, motor outcomes, neuropathology | Null | No further preclinical study | |
| Arroyo-Araujo et al., 2019 | Autism spectrum disorder | mGluR1 antagonist (JNJ16259685) | Behavioural activity | Positive | Not reported | |
| Jha et al., 2020 | Traumatic brain injury | Glibenclamide | Cognitive outcomes, biomarkers, motor outcomes, neuropathology | Glucose level, drug levels | Mixed across models and outcomes (positive, null, and negative) | Further preclinical testing |
| Kliewer et al., 2020 | Opioid-induced respiratory depression | Morphine | Respiratory rate | Constipation | Null | Not reported |
Risk of bias assessment of preclinical multilaboratory studies.
| Study | Sequence generation § | Baseline characteristics | Allocation concealment§ | Random housing | Blinding of personnel | Random outcome assessment | Blinding of outcome assessment | Incomplete outcome data*, § | Selective outcome reporting | Other sources of bias ¶ § |
|---|---|---|---|---|---|---|---|---|---|---|
| Reimer et al., 1985 | U* | U | L | U | H† | U | L | U | L | U |
| Crabbe et al., 1999 | U* | L | U | L | U | U | U | L | L | H |
| Alam et al., 2009 | U* | L | U | U | U | L | U | L | L | U |
| Spoerke et al., 2009 | U* | L | U | U | U | L | U | L | L | U |
| Jones et al., 2015 | L | L | U | U | L | L | L | L | L | L |
| Llovera et al., 2015 | L | L | U | U | L | L | L | L | L | L |
| Maysami et al., 2016 | H ‡ | H | L | U | L | L | L | L | H | H |
| Bramlett et al., 2016 | U* | U | U | U | L | L | L | U | L | H |
| Browning et al., 2016 | U* | U | U | U | L | L | L | U | L | H |
| Dixon et al., 2016 | U* | U | U | U | L | L | L | U | L | H |
| Gill et al., 2016 | H | L | U | U | U | L | L | U | U | L |
| Mountney et al., 2016 | U* | U | U | U | L | L | L | U | L | H |
| Shear et al., 2016 | U* | U | U | U | L | L | L | U | L | H |
| Arroyo-Araujo et al., 2019 | L | L | U | U | L | U | L | L | L | H |
| Jha et al., 2020 | U* | U | U | U | L | L | L | U | L | H |
| Kliewer et al., 2020 | U* | U | U | U | L | U | L | U | L | L |
-
Legend: H=High risk of bias (red), L=Low risk of bias (green), U=Unclear risk of bias (yellow).
-
Baseline Characteristics: Low risk = Relevant baseline characteristics equal between experimental groups or controlled for. Unclear = Relevant baseline characteristics are unreported. High risk = Relevant baseline characteristics unbalanced between experimental groups and not controlled.
-
Random Housing: Low risk = Animal cages were randomly placed within an animal room/facility, Unclear = Housing placement unreported, High risk = Animals placed in a non-random arrangement in animal room/facility.
-
Blinding of Outcome Assessment: Low risk = Outcome assessors were blinded to the study groups when assessing endpoints/animals Unclear = Insufficient information to determine if outcome assessors were blinded during an assessment. High Risk = Outcome assessors not blinded to the study groups.
-
Incomplete Outcome Data: Low risk = N values were consistent between methods and results for the outcomes. Unclear = N value was either not presented in the methods or in the results, and therefore there is insufficient information to permit judgment. High risk = N values were not consistent between methods and results for the outcomes.
-
Selective Reporting: Low risk = The methods section indicated pre-specified outcome measures. Unclear: Was not clear about the pre-specified primary endpoints and outcome results. High risk = The outcome was presented in the results but not pre-specified in the methods section.
-
*
Method of randomization not specified.
-
†
Assessed as high because one arm of the study was inadvertently unblinded.
-
‡
Some labs used appropriate randomization where others used pseudo-randomization.
-
§
Items in agreement with the Cochrane Risk of Bias tool.
-
¶
other sources include funding influences, conflicts of interest, contamination, a unit of analysis errors.
Comparison of characteristics between single lab and multilaboratory studies.
| Multilaboratory studies (n=16) | Single lab studies (n=100) | |
|---|---|---|
| Median sample size (range) | 111 (23–384) | 19 (10–72) |
| Total animals used | 2,145 | 2,166 |
| Publication date range | 1985–2020 | 1980–2019 |
| Disease model | n (%) | n (%) |
| TBI | 6 (38) | 46 (46) |
| Myocardial infarction | 2 (13) | 20 (20) |
| Stroke | 2 (13) | 16 (16) |
| Traumatic injury | 2 (13) | 10 (10) |
| Stimulant exposure | 2 (13) | NA |
| Diabetes | 1 (6) | 2 (2) |
| Autism spectrum disorder | 1 (6) | 6 (6) |
| Animal species | n (%) | n (%) |
| Rat | 7 (44) | 42 (42) |
| Mouse | 6 (38) | 31 (31) |
| Swine | 3 (19) | 12 (12) |
| Rabbit | 1 (6) | 3 (3) |
| Dog | 1 (6) | 9 (9) |
| Monkey | 0 | 2 (2) |
| Cat | 0 | 1 (1) |
| Animal sex | n (%) | n (%) |
| Male | 10 (63) | 69 (69) |
| Female | 2 (13) | 13 (13) |
| Both | 3 (19) | 12 (12) |
| Not reported | 1 (6) | 6 (6) |
| Quality domain | Percent of studies that performed each measure (%) | |
| Randomization | 94 | 57 |
| Randomization methods | 19 | 7 |
| Blinding of personnel | 69 | 24 |
| Blinding of outcome assessment | 75 | 53 |
| Complete outcome data | 38 | 38 |
Statements of future recommendations.
| Author, Year | Recommendation statements |
|---|---|
| Reimer et al., 1985 | Nothing reported |
| Crabbe et al., 1999 | Relatively small genetic effects should first be replicated locally before drawing conclusions... genotypes should be tested in multiple labs and evaluated with multiple tests of a single behavioral domain |
| Alam et al., 2009 | Based upon the findings of the current study that demonstrated the impressive hemostatic properties of plasma, we have proceeded to successfully develop and test (in the same model) lyophilized [freeze dried plasma]. |
| Spoerke et al., 2009 | The species-specific differences in factor activities will require ongoing investigation to ensure full safety and efficacy. Our future investigations will include a comprehensive evaluation of the effects of the lyophilization process on coagulation properties of the LP. |
| Jones et al., 2015 | other investigators can adopt the protocols [for measuring infarct size in mice, rabbits, and pigs in a manner that is rigorous, accurate, and reproducible] in their own laboratories. |
| Llovera et al., 2015 | future clinical trials testing immunotherapeutic drugs for stroke will need to ensure that the included study population feature a substantial neuroinflammatory reaction to the brain injury |
| Maysami et al., 2016 | interleukin 1 receptor antagonist should be evaluated in more extensive clinical stroke trials |
| Bramlett et al., 2016 | Although we cannot rule out the possibility that other doses or more prolonged treatment could show different effects, the lack of efficacy of EPO reduced enthusiasm for its further investigation in OBTT. |
| Browning et al., 2016 | …need for OBTT to study LEV further. This includes studies of dose response, therapeutic window, mechanism, and testing in our large animal FPI model in micropigs… consider a randomized controlled trial examining early administration in patients |
| Dixon et al., 2016 | Our findings reduce enthusiasm for further investigation of this therapy in OBTT and suggest that if this strategy is to be pursued further, alternative CsA analogs with reduced toxicity should be used. |
| Gill et al., 2016 | …pause in proceeding with clinical trials without further preclinical testing. |
| Mountney et al., 2016 | the current findings do not support the beneficial effects of simvastatin… it will not be further pursued by OBTT. |
| Shear et al., 2016 | The marginal benefits achieved with nicotinamide, however, which appeared sporadically across the TBI models, has reduced enthusiasm for further investigation by the OBTT Consortium. |
| Arroyo-Araujo et al., 2019 | Nothing reported |
| Jha et al., 2020 | Optimizing [GLY] treatment regimens (dose, duration, timing), surrogate markers for edema subtypes on MRI, pathway-specific biomarkers, and genetic risk stratification may facilitate precision medicine and patient selection for future clinical trials. |
| Kliewer et al., 2020 | Nothing reported |
-
Legend: FDP – Freeze-dried plasma; LP – Lyophilized plasma; EPO – Erythropietin; OBTT – Operation Brain Trauma Therapy; LEV – Levetiracetam; FPI – Fluid percussion brain injury; CsA – cyclosporin-A; cyclosporine; TBI – Traumatic Brain Injury; GLY - Glibenclamide.
Risk of bias for other sources of bias.
| Study | Funding influences | Conflicts of interest | Contamination | Unit of analysis errors |
|---|---|---|---|---|
| Reimer et al., 1985 | L | U | L | U |
| Crabbe et al., 1999 | L | U | L | H |
| Alam et al., 2009 | L | U* | L | U |
| Spoerke et al., 2009 | L | U* | L | U |
| Jones et al., 2015 | L | L | L | L |
| Llovera et al., 2015 | L | L | L | U |
| Maysami et al., 2016 | L | H | L | U |
| Bramlett et al., 2016 | L | H | L | U |
| Browning et al., 2016 | L | H | L | U |
| Dixon et al., 2016 | L | H | L | U |
| Gill et al., 2016 | L | L | L | U |
| Mountney et al., 2016 | L | H | L | U |
| Shear et al., 2016 | L | H | L | U |
| Arroyo-Araujo et al., 2019 | H | H | L | L |
| Jha et al., 2020 | L | H | L | U |
| Kliewer et al., 2020 | L | L | L | U |
-
*
financial disclosure, no statement of other conflicts provided.
Frequency of reported preclinical multilaboratory checklist items.
| Domain | # | Item Description | % of studies that reported |
|---|---|---|---|
| Intro/ abstract | 1 | Identification as a multicenter/multilaboratory study in title | 38 |
| 2 | Abstract states number of participating centers | 50 | |
| Standards | 3 | Community based reporting guidelines listed | 13 |
| 4 | Names of each participating center listed | 100 | |
| 5 | List roles of participating centers (central coordinating center, experimental site) | 88 | |
| 6 | No changes, or if applicable major changes to study protocol after commencement are documented | 94 | |
| Replicates (biological vs. technical) | 7 | Results substantiated by repetition under a range of conditions at each site | 100 |
| 8 | Number of subjects per outcome | 100 | |
| 9 | Number of measurements per subject for one experimental outcome stated | 75 | |
| 10 | Number of subjects per lab | 81 | |
| Statistics | 11 | List of the total number of subjects used in each experimental group | 81 |
| 12 | List of all statistical tests used | 100 | |
| 13 | Definition of the measure of central tendency | 100 | |
| 14 | Definition of the measure of dispersion | 100 | |
| Randomization | 15 | Random group assignment reported | 100 |
| 16 | Description of the method of random group assignment | 31 | |
| Blinding | 17 | Experimenters blinded to group allocation during conduct of the experiment | 75 |
| 18 | Experimenters blinded to group allocation during result assessment | 75 | |
| Sample Size Estimation | 19 | Description of an a priori primary outcome | 94 |
| 20 | Sample size for each site computed during study design | 31 | |
| 21 | Description of the method of sample size determination | 31 | |
| Inclusion and Exclusion Criteria | 22 | Total number of animals for the experiment reported | 88 |
| 23 | Description of the criteria used for the exclusion of any data or subjects | 50 | |
| 24 | List losses and exclusions of animals at the end of experiment | 50 | |
| 25 | All outcomes described, or description of any outcomes that were measured and not reported in the results section | 100 | |
| 26 | Previous or pilot/preliminary studies performed and listed | 88 | |
| 27 | Results were significant, or if not, null or negative outcomes included in the results | 100 | |
| Discussion | 28 | Limitations of the study are documented | 75 |
| 29 | Discrepancies in results across labs expected or absent, or if not, they discussed | 100 |
-
Legend: Coloured cells indicate the frequency (%) of item reported over all included studies. Frequency (%) ranges: 0-37 = red; 38-76 = yellow; 77-100 = green.
Preclinical single lab studies selection process for the comparison.
| Reimer et al., 1985 | Spoerke et al., 2009 | Alam et al., 2009 | Llovera et al., 2015 | Maysami et al., 2016 | Gill et al., 2016 | Bramlett et al., 2016 | Browning et al., 2016 | Dixon, 2016 | Mountney et al., 2016 | Shear et al., 2016 | Arroyo-Araujo et al., 2019 | Jha et al., 2020 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Records identified/ abstracts screened | 71 | 189 | 61 | 52 | 33 | 39 | 177 | 31 | 197 | 45 | 28 | 26 | 29 |
| Full-texts assessed for eligibility | 29 | 22 | 18 | 14 | 21 | 11 | 57 | 15 | 48 | 30 | 18 | 12 | 10 |
| Records considered eligible | 13 | 5 | 5 | 6 | 10 | 2 | 1 SR | 4 | 8 | 9 | 12 | 6 | 5 |
| Records used for comparison | 10 | 5 | 5 | 6 | 10 | 2 | 1 SR | 4 | 8 | 9 | 10 | 6 | 5 |
Multilaboratory reporting checklist with item domain and source(s).
| Domain | # | Item Description | Source(s) |
|---|---|---|---|
| Intro/abstract | 1 | Identification as a multicenter/multilaboratory study in title | CONSORT |
| 2 | Abstract states number of participating centers | CONSORT | |
| Standards | 3 | Community based reporting guidelines listed | NIH |
| 4 | Names of each participating center listed | GCP E6(R2) | |
| 5 | List roles of participating centers (central coordinating center, experimental site) | GCP E6(R2) | |
| 6 | No changes, or if applicable major changes to study protocol after commencement are documented | CONSORT | |
| Replicates (biological vs. technical) | 7 | Results substantiated by repetition under a range of conditions at each site | NIH, CONSORT |
| 8 | Number of subjects per outcome | NIH, CONSORT | |
| 9 | Number of measurements per subject for one experimental outcome stated | NIH, CONSORT | |
| 10 | Number of subjects per lab | GCP E6(R2) | |
| Statistics | 11 | List of the total number of subjects used in each experimental group | NIH, CONSORT |
| 12 | List of all statistical tests used | NIH, CONSORT | |
| 13 | Definition of the measure of central tendency | NIH | |
| 14 | Definition of the measure of dispersion | NIH | |
| Randomization | 15 | Random group assignment reported | NIH, CONSORT |
| 16 | Description of the method of random group assignment | NIH, CONSORT | |
| Blinding | 17 | Experimenters blinded to group allocation during conduct of the experiment | NIH, CONSORT |
| 18 | Experimenters blinded to group allocation during result assessment | NIH, CONSORT | |
| Sample Size Estimation | 19 | Description of an a priori primary outcome | CONSORT |
| 20 | Sample size computed during study design | NIH, CONSORT | |
| 21 | Description of the method of sample size determination | NIH, CONSORT | |
| Inclusion and Exclusion Criteria | 22 | Total number of animals for the experiment reported | GCP E6(R2) |
| 23 | Description of the criteria used for the exclusion of any data or subjects | NIH, CONSORT | |
| 24 | List losses and exclusions of animals at the end of experiment | CONSORT | |
| 25 | All outcomes described, or description of any outcomes measured but not reported in results | NIH, CONSORT | |
| 26 | Previous or pilot/preliminary studies performed and listed | NIH | |
| 27 | Results were significant, or if not, null or negative outcomes included in the results | NIH | |
| Discussion | 28 | Limitations of the study are documented | CONSORT |
| 29 | Discrepancies in results across labs expected or absent, or if not, they discussed | CONSORT |
Additional files
-
MDAR checklist
- https://cdn.elifesciences.org/articles/76300/elife-76300-mdarchecklist1-v2.docx
-
Supplementary file 1
Preclinical multilaboratory search strategy.
- https://cdn.elifesciences.org/articles/76300/elife-76300-supp1-v2.docx
-
Supplementary file 2
PRESS review of search strategy.
- https://cdn.elifesciences.org/articles/76300/elife-76300-supp2-v2.docx
-
Supplementary file 3
Completeness of reporting of preclinical multilaboratory studies for 29 reporting items.
- https://cdn.elifesciences.org/articles/76300/elife-76300-supp3-v2.docx
-
Supplementary file 4
Quality scores, effect sizes, and effect size ratios of multilaboratory and single lab studies.
- https://cdn.elifesciences.org/articles/76300/elife-76300-supp4-v2.docx
-
Reporting standard 1
PRISMA Checklist.
- https://cdn.elifesciences.org/articles/76300/elife-76300-repstand1-v2.docx