Biomarkers: Getting closer to the clinic
Note added [December 5, 2023]
This Insight article has been updated to reflect a correction made to the associated Research Article: in the sixth paragraph, the number of connections between EpiScores and 11 diseases or death has been changed from 137 to 130.
Lengthening life expectancies and decreased mortality rates have led to an unprecedented expansion of the older population. At the same time, chronic diseases – which often affect older individuals – have become more prevalent. Healthcare systems around the world are falling short of this challenge, in part because they remain focused on preventing and curing one disease at a time, even though 80% of clinical patients over 60 have multiple diseases at once.
Even when one specific disease causes most of a person’s symptoms, older patients often have co-existing conditions that affect the course, treatment and prognosis of the main disease. Pressed for time, physicians often ignore underlying illnesses until they begin to seriously affect the patient’s health or start causing frailty. There is no easy solution to this rising crisis, but the emerging field of biomarkers may soon come to the aid of clinicians.
Biomarkers are molecules, genes or characteristics that can be used to detect or predict the onset of a disease. Traditionally, biomarkers have included circulating levels of plasma proteins, lipids and other metabolites. More recently, epigenetic markers – chemical modifications of DNA that affect whether genes are turned on or off, such as addition of methyl groups at specific DNA sites – have shown promise as biomarkers for age-related conditions. Using biomarkers could allow physicians to obtain a molecular map of a patient’s health from a single drop of blood. This would allow clinicians to detect illnesses before they become symptomatic, which is particularly important in the case of serious conditions that could become chronic (Tanaka et al., 2020).
Developing algorithms that extract the relevant information from biomarkers in the blood is perhaps the most promising and potentially powerful line of research in chronic diseases. Until recently, most biomarker studies examined one layer of information (DNA modifications, protein levels or specific metabolites) at a time. However, combining information on DNA methylation with the level of a small number of circulating proteins has been shown to predict the risk of specific chronic diseases as well as global, adverse health outcomes such as having several illnesses at once, and mortality (Lu et al., 2019; Levine et al., 2018; Belsky et al., 2020). Now, in eLife, Riccardo Marioni from the University of Edinburgh and colleagues – including Danni Gadd, Robert Hillary, Daniel McCartney and Shaza Zaghlool as joint first authors – report on how to leverage the associations between DNA methylation and protein levels to predict the onset of disease earlier and more accurately (Gadd et al., 2022).
The team (who are based in the United Kingdom, the United States, Germany, Australia and Qatar) first measured the abundance of 953 proteins in the blood plasma of people in the German KORA cohort (an epidemiological study that ran from 1984 to 2001 in Augsburg and evaluated participants every five years, with an emphasis on major chronic diseases) and the Scottish Lothian Birth Cohort 1936 (the surviving participants of the Scottish Mental Survey 1947 who now live in the Lothian area of Scotland). Gadd et al. then used machine learning to identify clusters of specific DNA methylation sites that could predict the levels of each protein in the plasma. This data was used to assign an epigenetic score or ‘EpiScore’ to each protein. Using this approach, Gadd et al. found that their new algorithm could predict between 1% and 58% of the variation between different people in the plasma levels of 109 proteins.
Next, the team applied the EpiScores of the 109 proteins to data from an independent epidemiological study called Generation Scotland to test whether it was possible to predict the onset of 11 major chronic diseases, as well as death, over a follow-up period of 14 years (Figure 1). This resulted in the identification of 130 connections between EpiScores and 11 diseases or death. Some EpiScores predicted the onset of selected conditions but other were associated with multiple conditions and, perhaps unsurprisingly, the results also suggested a strong correlation between inflammation and age-related chronic disease.
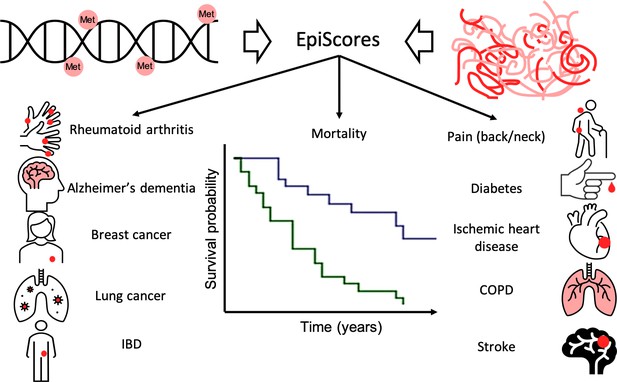
Epigenetic scores of plasma proteins predict onset of major chronic diseases over 14 years.
A machine learning approach was used to find associations, called EpiScores, between DNA methylation (top left) and the abundance of 953 plasma proteins (top right). The results identified 109 proteins with EpiScores that explained between 1% and 58% of the variance in their levels. These scores were then applied to an epidemiological study that contains the medical records of 1,537 individuals over the course of 14 years. Gadd et al. found 130 connections between these EpiScores and 11 age-related conditions (represented by icons), and also between the EpiScores and mortality (represented by the survival graph).
One of the notable observations (that has also been reported in previous studies) is that these analyses on EpiScores confirmed known associations between certain proteins and diseases, even when there is only a moderate correlation between the EpiScore and the protein. This suggests that EpiScores are not a mere proxy for plasma protein levels, but may contain different information about disease risk. In the future, it is likely that biomarkers for disease will encompass multiple molecular layers, such as protein levels together with epigenetic markers or metabolite composition.
The findings of Gadd et al. offer a glimpse into a possible future of medicine. One could imagine a busy physician evaluating a 75-year-old patient complaining of sudden back pain. The physician collects a small blood sample and analyzes it using a fast robotized laboratory connected to a powerful computer that can measure molecular biomarkers and assign a ‘health score’. The computer would then provide information about the patient’s risk for potential diseases that the physician can address before they become symptomatic. The systematic use of this technology could increase awareness and understanding of co-existing, but not yet visible, medical problems.
Of course, before this can happen more research is needed. The predictivity of some EpiScores is modest and only adequate for risk prediction. Even in this context, it would be important to understand whether performing early interventions on patients with high scores is cost effective. As always in prevention, there is a trade-off between the stigmata of tagging an individual as ‘high risk’ and how this information can be used to improve health. A study in which information about proteins and DNA methylation is first compared ‘head to head’ in the same large cohort, and then combined, could reveal whether these two biomarkers provide complementary information and increase specificity. Over time, the data collected systematically using this approach and surveillance studies of electronic medical records could help identify common co-morbidities, allowing clinicians to develop more effective strategies for treating patients with complex combinations of diseases.
References
Article and author information
Author details
Publication history
- Version of Record published: February 25, 2022 (version 1)
Copyright
This is an open-access article, free of all copyright, and may be freely reproduced, distributed, transmitted, modified, built upon, or otherwise used by anyone for any lawful purpose. The work is made available under the Creative Commons CC0 public domain dedication.
Metrics
-
- 739
- views
-
- 78
- downloads
-
- 3
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Epidemiology and Global Health
We discuss 12 misperceptions, misstatements, or mistakes concerning the use of covariates in observational or nonrandomized research. Additionally, we offer advice to help investigators, editors, reviewers, and readers make more informed decisions about conducting and interpreting research where the influence of covariates may be at issue. We primarily address misperceptions in the context of statistical management of the covariates through various forms of modeling, although we also emphasize design and model or variable selection. Other approaches to addressing the effects of covariates, including matching, have logical extensions from what we discuss here but are not dwelled upon heavily. The misperceptions, misstatements, or mistakes we discuss include accurate representation of covariates, effects of measurement error, overreliance on covariate categorization, underestimation of power loss when controlling for covariates, misinterpretation of significance in statistical models, and misconceptions about confounding variables, selecting on a collider, and p value interpretations in covariate-inclusive analyses. This condensed overview serves to correct common errors and improve research quality in general and in nutrition research specifically.
-
- Ecology
- Epidemiology and Global Health
Zoonotic disease dynamics in wildlife hosts are rarely quantified at macroecological scales due to the lack of systematic surveys. Non-human primates (NHPs) host Plasmodium knowlesi, a zoonotic malaria of public health concern and the main barrier to malaria elimination in Southeast Asia. Understanding of regional P. knowlesi infection dynamics in wildlife is limited. Here, we systematically assemble reports of NHP P. knowlesi and investigate geographic determinants of prevalence in reservoir species. Meta-analysis of 6322 NHPs from 148 sites reveals that prevalence is heterogeneous across Southeast Asia, with low overall prevalence and high estimates for Malaysian Borneo. We find that regions exhibiting higher prevalence in NHPs overlap with human infection hotspots. In wildlife and humans, parasite transmission is linked to land conversion and fragmentation. By assembling remote sensing data and fitting statistical models to prevalence at multiple spatial scales, we identify novel relationships between P. knowlesi in NHPs and forest fragmentation. This suggests that higher prevalence may be contingent on habitat complexity, which would begin to explain observed geographic variation in parasite burden. These findings address critical gaps in understanding regional P. knowlesi epidemiology and indicate that prevalence in simian reservoirs may be a key spatial driver of human spillover risk.






