Genetic Code: Expanding codon size
Cells use a genetic code to translate the information contained in DNA and RNA sequences into the amino-acid building blocks that make up a protein (Nirenberg et al., 1965; Söll et al., 1965). DNA molecules are chain-like structures consisting of two entwined strands that encode information using an ‘alphabet’ of four nucleotide building blocks (made up of a sugar and a phosphate group, and one of four nitrogenous bases, A, C, G and T).
Some segments of the DNA genome are instructions for making proteins, and a messenger RNA molecule (mRNA) is produced from the DNA template for each protein-coding gene. On the ribosome, molecules known as transfer RNAs (tRNAs) decode the information in mRNAs by reading it in three-letter groups, also known as codons, allowing for 64 unique triplet combinations (three of which are used as stop signals). Despite this wealth of codons, most organisms usually use just 20 amino acids, making the code redundant as several codons can code for the same amino acid.
Expanding or modifying the genetic code may enable scientists to use cells as factories for making an array of molecules, which could further therapies based on proteins, and in even grander schemes, to enable the creation of artificial life forms. Recent engineering efforts have successfully produced proteins using 22 or even 23 different amino acids, rather than the usual 20 (Wright et al., 2018; Tharp et al., 2021). However, the standard triplet codons cannot be easily reassigned into new amino acids, because even though decoding the 64 codons allows for redundancy, all codons are assigned to a specific amino acid in an organism. Reassigning a codon to a new amino acid would drastically change the organisms’ protein composition.
A quadruplet system based on four-letter codons rather than three has 256 total codons that could encode many more amino acids, independent of the natural triplet codon system. Expanding the number of protein building blocks would help to produce highly specialized proteins containing unnatural amino acids, potentially opening the door to advances in both basic biology and therapeutic applications (Hohsaka et al., 1996).
One approach to expanding the genetic code is based on a natural process called +1 frameshifting, where four rather than three nucleotide bases are effectively decoded as a single amino acid (Riyasaty and Atkins, 1968). The insertion of a single base generates a frameshift in an otherwise triplet codon gene, shifting the frame by one letter. So-called frameshift suppressor tRNAs allow protein synthesis to continue past this insertion to produce a normal full-length protein. For example, some tRNAs can read frameshift mutations, including insertions or deletions of one or two nucleotides in the mRNA. A tRNA that suppresses a +1 frameshift effectively reads or decodes a quadruplet codon (Figure 1).
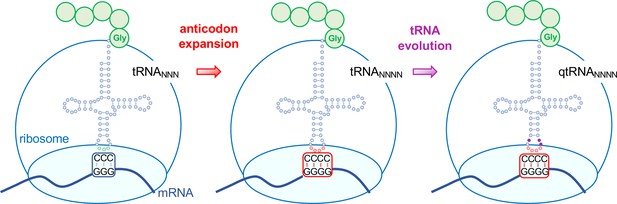
Evolving tRNAs to efficiently read quadruplet codons.
The schematic illustrates the approach used by DeBenedictis et al. to evolve triplet-decoding tRNAs (left) into tRNAs with expanded anticodon loops (red dots) to decode quadruplet codons consisting of four nucleotide bases (N; middle), and ultimately into efficient quadruplet codon decoders (right). To test the efficiency of four-base translation, DeBenedictis et al. created reporter genes (such as luciferase) with a single base insertion (also referred to as +1 frameshift). The ability of a particular tRNA variant to read that +1-frameshift mutation as a four-base codon can be measured as a function of how much full length and active reporter protein (e.g., luciferase) is made in cells. DeBenedictis et al. then recorded the translation efficiency of tRNAs with simple mutations to expand the anticodon loop (middle, red dots). Next, tRNAs were evolved through various mutations into more effective quadruplet-decoding tRNAs (qtRNAs, purple dots, right). The work represents an important step towards engineering a quadruplet genetic code with 256 codons.
Image credit: Tarana Siddika, Ilka U. Heinemann, Patrick O’Donoghue (CC BY 4.0).
Translation using quadruplet codons exists in both natural and synthetic systems, so why did nature favor a triplet system overall? Now, in eLife, Erika DeBenedictis, Dieter Söll and Kevin Esvelt at the Massachusetts Institute of Technology and Yale University, report how tRNAs can be evolved to read quadruplet codons more efficiently (DeBenedictis et al., 2022). DeBenedictis et al. made simple mutations to several natural and synthetic tRNAs to generate various quadruplet-decoding RNAs (qtRNAs) based on the natural frameshifting variants and tested their efficiency in the bacterium Escherichia coli. This revealed that quadruplet codons only had a translation efficiency of 1%–3% compared to triplet codons. Some qtRNAs also showed significantly slowed growth rates in E. coli, while others were better tolerated despite their ability to translate quadruplet codons.
DeBenedictis et al. then tested whether tRNAs could evolve into qtRNAs through replacement of the entire anticodon (the complementary sequence to the codon located on the tRNA) with quadruplet codons using a phage-based library selection approach. This involved encoding modified qtRNAs into the genome of bacteriophages, which in turn infected the E. coli bacteria. Only functional qtRNAs enabled phages to successfully reproduce, thus highlighting effective quadruplet codon/anticodon pairs and comparing the efficiency of quadruplet decoding. Their data revealed that functional qtRNAS that can successfully decode quadruplet codons can arise through just a few mutations.
Next, the positions surrounding the anticodon were mutated across a large library of different qtRNAs (Figure 1). These experiments identified qtRNA variants that displayed up to 40-fold improvement in quadruplet decoding efficiency compared to unmutated quadruplet decoding tRNAs. Finally, mass spectrometry was used to show how some qtRNAs provided ‘high-fidelity’ four-base decoding and inserted just a single type of amino acid in response to a quadruplet codon. Other qtRNAs were less selective and the same four-base codon was ‘read’ with multiple different amino acids, revealing ambiguity as a potential limitation in quadruplet decoding.
Overall, the findings highlight the potential and limitations of a genetic code based on quadruplet codons. Quadruplet codons can be used to produce highly specialized proteins with new functionalities, but low translation fidelity and limited efficiency remain challenges in the field. DeBenedictis et al. use multiple approaches to mutagenize tRNAs, and they generated many improved qtRNA variants that represent a full palate of starting points to reassign quadruplet codons to new amino acids. The researchers demonstrated the power of tRNA variations on their own, and significant improvements in quadruplet decoding by engineering tRNAs alone. Combining qtRNAs with engineered tRNA synthetases to improve incorporation of unnatural amino acids (reviewed in Kim et al., 2022) or with genetically modified ribosomes to enhance 4-base translation (Dunkelmann et al., 2021) could further improve efficiency and decrease ambiguity in translating proteins with an increased codon size, thus vastly expanding the genetic code.
The work of DeBenedictis et al. points towards several areas worth further investigation to improve translation efficiency and fidelity with quadruplet codons and they show that in-roads can be made in both areas though tRNA engineering. Together with recent efforts to use quadruplet codons to encode synthetic amino acids (DeBenedictis et al., 2021; Dunkelmann et al., 2021), the current study suggests that the expansion of the genetic code with the help of quadruplet codons may be within reach.
References
-
Incorporation of nonnatural amino acids into streptavidin through in vitro frame-shift suppressionJournal of the American Chemical Society 118:9778–9779.https://doi.org/10.1021/ja9614225
-
Engineering translation components for genetic code expansionJournal of Molecular Biology 434:167302.https://doi.org/10.1016/j.jmb.2021.167302
-
External suppression of a frameshift mutant in salmonellaJournal of Molecular Biology 34:541–557.https://doi.org/10.1016/0022-2836(68)90179-4
-
Acetylation regulates thioredoxin reductase oligomerization and activityAntioxidants & Redox Signaling 29:377–388.https://doi.org/10.1089/ars.2017.7082
Article and author information
Author details
Publication history
- Version of Record published: May 11, 2022 (version 1)
Copyright
© 2022, Siddika et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 1,949
- views
-
- 179
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Biochemistry and Chemical Biology
- Cell Biology
Hibernation is a period of metabolic suppression utilized by many small and large mammal species to survive during winter periods. As the underlying cellular and molecular mechanisms remain incompletely understood, our study aimed to determine whether skeletal muscle myosin and its metabolic efficiency undergo alterations during hibernation to optimize energy utilization. We isolated muscle fibers from small hibernators, Ictidomys tridecemlineatus and Eliomys quercinus and larger hibernators, Ursus arctos and Ursus americanus. We then conducted loaded Mant-ATP chase experiments alongside X-ray diffraction to measure resting myosin dynamics and its ATP demand. In parallel, we performed multiple proteomics analyses. Our results showed a preservation of myosin structure in U. arctos and U. americanus during hibernation, whilst in I. tridecemlineatus and E. quercinus, changes in myosin metabolic states during torpor unexpectedly led to higher levels in energy expenditure of type II, fast-twitch muscle fibers at ambient lab temperatures (20 °C). Upon repeating loaded Mant-ATP chase experiments at 8 °C (near the body temperature of torpid animals), we found that myosin ATP consumption in type II muscle fibers was reduced by 77–107% during torpor compared to active periods. Additionally, we observed Myh2 hyper-phosphorylation during torpor in I. tridecemilineatus, which was predicted to stabilize the myosin molecule. This may act as a potential molecular mechanism mitigating myosin-associated increases in skeletal muscle energy expenditure during periods of torpor in response to cold exposure. Altogether, we demonstrate that resting myosin is altered in hibernating mammals, contributing to significant changes to the ATP consumption of skeletal muscle. Additionally, we observe that it is further altered in response to cold exposure and highlight myosin as a potentially contributor to skeletal muscle non-shivering thermogenesis.
-
- Biochemistry and Chemical Biology
- Neuroscience
In most mammals, conspecific chemosensory communication relies on semiochemical release within complex bodily secretions and subsequent stimulus detection by the vomeronasal organ (VNO). Urine, a rich source of ethologically relevant chemosignals, conveys detailed information about sex, social hierarchy, health, and reproductive state, which becomes accessible to a conspecific via vomeronasal sampling. So far, however, numerous aspects of social chemosignaling along the vomeronasal pathway remain unclear. Moreover, since virtually all research on vomeronasal physiology is based on secretions derived from inbred laboratory mice, it remains uncertain whether such stimuli provide a true representation of potentially more relevant cues found in the wild. Here, we combine a robust low-noise VNO activity assay with comparative molecular profiling of sex- and strain-specific mouse urine samples from two inbred laboratory strains as well as from wild mice. With comprehensive molecular portraits of these secretions, VNO activity analysis now enables us to (i) assess whether and, if so, how much sex/strain-selective ‘raw’ chemical information in urine is accessible via vomeronasal sampling; (ii) identify which chemicals exhibit sufficient discriminatory power to signal an animal’s sex, strain, or both; (iii) determine the extent to which wild mouse secretions are unique; and (iv) analyze whether vomeronasal response profiles differ between strains. We report both sex- and, in particular, strain-selective VNO representations of chemical information. Within the urinary ‘secretome’, both volatile compounds and proteins exhibit sufficient discriminative power to provide sex- and strain-specific molecular fingerprints. While total protein amount is substantially enriched in male urine, females secrete a larger variety at overall comparatively low concentrations. Surprisingly, the molecular spectrum of wild mouse urine does not dramatically exceed that of inbred strains. Finally, vomeronasal response profiles differ between C57BL/6 and BALB/c animals, with particularly disparate representations of female semiochemicals.






