Coronary artery established through amniote evolution
Figures
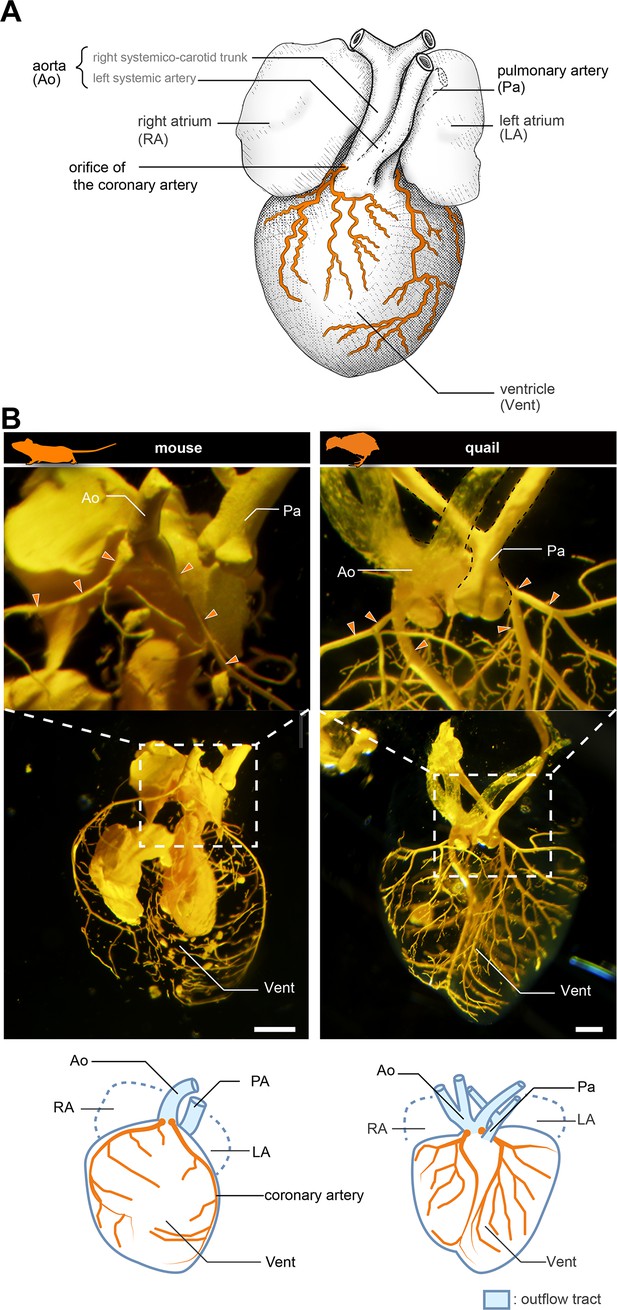
Anatomy of coronary arteries of amniotes.
(A) Scheme of lizard coronary artery based on the data for the lace monitor (Varanus varius). In this lizard, a single coronary artery originates from the root of the right systemicocarotid trunk (the aorta in mammals) close to the heart and bifurcates almost at its point of origin to form a ventral and a dorsal division. The figure is based on MacKinnon and Heatwole, 1981, with some modifications for clarity: we drew shadings and added the position of the pulmonary artery. (B) Resin-injected coronary vessels of the mouse (17.5 dpc) and quail (stage 28) embryos. Scale bars: 1 mm.

Development of murine coronary artery visualized in latex-injected fetuses.
The right panels are the partial enlargements of the left panels. The blue arrow shows the coronary vein. Scale bars: 500 µm.
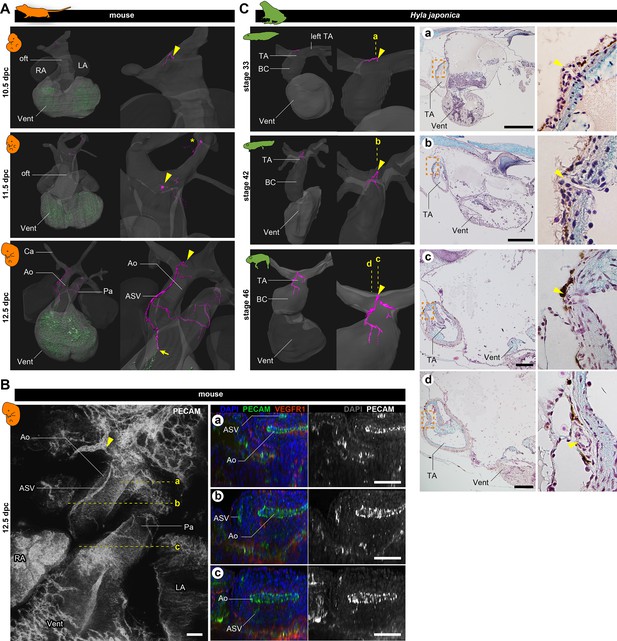
Development of vasculature in the outflow tract of mice and frogs.
(A) Ventral views of three-dimensional reconstructed images of murine hearts. We visualized two types of primitive blood vessels: aortic subepicardial vessels (ASVs; pink) and primitive coronary plexuses (green). Although a plexus of vessels surrounded the ASVs, we visualized only the thickest vessels connected to the orifice (pink, indicated with arrowheads) to facilitate recognition. The ASV orifices were located at the bifurcation point of the outflow tract. We also found an extra orifice in the left carotid artery at 11.5 dpc (asterisk). At 12.5 dpc, the peripheral end of the ASV formed an anastomosis with the primitive coronary plexus at the outflow tract–ventricle boundary (arrow). Blood vessels were visualized using immunohistochemical staining for PECAM-1 (panel B and Figure 2—figure supplement 1). (B) Fluorescence images used to construct the three-dimensional images in (A). Transverse sections at the levels a–c in the left panel are shown in the right panels. The peripheral end of the ASV merged into the aorta (c). (C) Ventral views of three-dimensional reconstructed images of the hearts of the Japanese tree frog (Hyla japonica) constructed from histological sections show small vessels located on the outflow tract with orifices at the same location as in mice (arrowheads). No primitive coronary plexuses were found. Ao, aorta; BC, bulbus cordis; Ca, carotid artery; LA, left atrium; oft, outflow tract; Pa, pulmonary artery; RA, right atrium; TA, truncus arteriosus; Vent, ventricle. Scale bars: 500 µm.
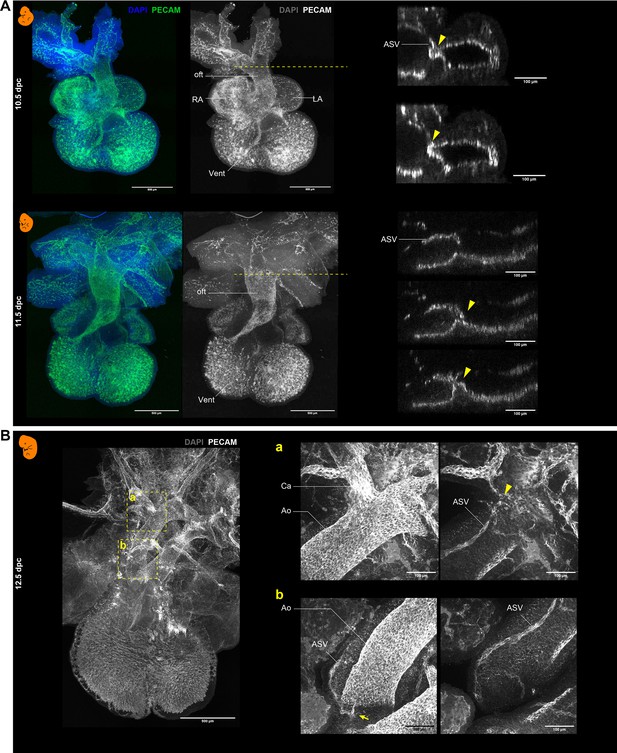
Whole-mount immunohistochemistry with PECAM-1 in mouse embryos.
(A) Hearts at 10.5 and 11.5 dpc. Right panels are the transverse sections at the levels (dashed lines) of the ASV orifices (arrowheads). (B) Heart at 12.5 dpc. Panels a and b show images taken at different depths. The arrowhead and the arrow indicate the positions of the primary and secondary orifices, respectively. Also see Figure 2A and B. Ao, aorta; Ca, carotid artery; LA, left atrium; RA, right atrium; oft, outflow tract; Vent, ventricle.
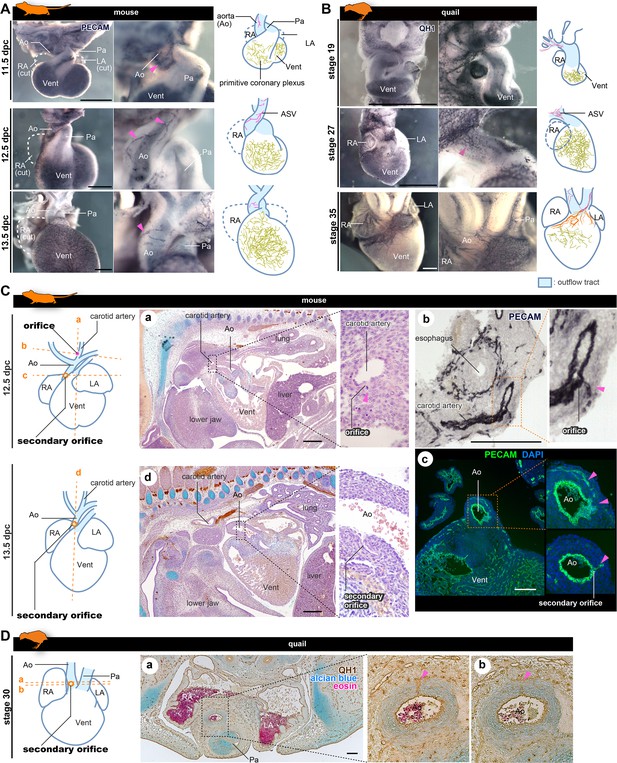
The positions of the orifices of the coronary arteries.
(A, B) ASVs and primitive coronary plexuses are present in the embryonic stages in mice (A) and quails (B). The blood vessels were visualized by immunohistochemistry. Pink arrowheads indicate ASVs originated from the cranial part of the outflow tracts (same to the following panels). (C) ASVs in 12.5 dpc mouse embryos branched from the root of the future carotid artery (a). Immunohistochemical staining of cryosections shows two orifices: at the root of the carotid artery (b) and at the boundary of the outflow tract and ventricle (c). In 13.5 dpc embryos, the secondary orifice developed at the aortic root instead of the carotid artery orifice, which was lost (d). (D) In quails at stage 30, the secondary orifice was formed at the aortic root, as in mice. Immunohistochemical and HE staining images of cryosections (a and b). Ao, aorta; LA, left atrium; RA, right atrium; Vent, ventricle. Scale bars: 500 µm (A, B), 100 µm (C, D).
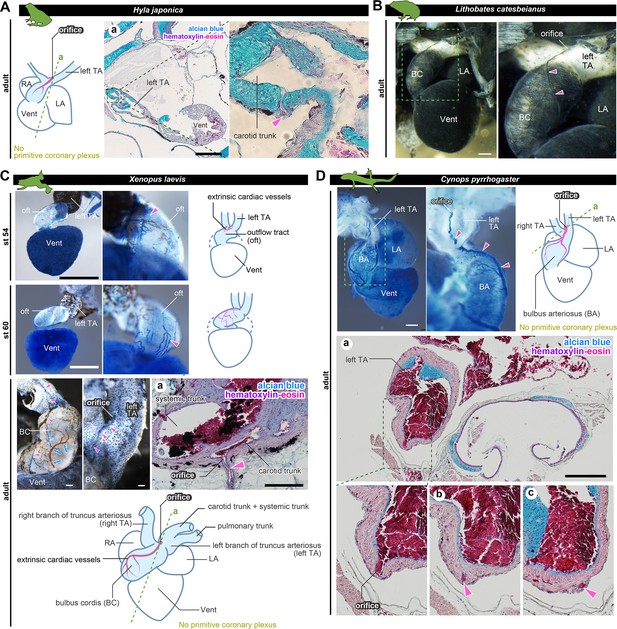
The positions of the orifices of the coronary arteries.
(A) In the Japanese tree frog (Hyla japonica), the ASV-like vessels (pink arrowheads) branched from the aortic trunk close to the root of the carotid artery. (B) Ink-injected heart of African bullfrog (Lithobates catesbeianus; tadpole). (C) Development of the extrinsic cardiac arteries of Xenopus laevis. Ink injection visualized the blood vessels. In adult X. laevis, the ASV-like vessels branched from the carotid artery in the aortic trunk (a). (D) Ink-injected heart of the adult Japanese fire-belly newt (Cynops pyrrhogaster). The boxed area in the left image is enlarged and slightly rotated so that the distribution of blood vessels can be easily seen. Histological sections (a–c) show that the ASV-like vessels had an orifice at the root of the carotid artery (a), and it continued in the outflow tract (b, c). Arrowheads indicate extrinsic arteries on the outflow tract. Scale bars: 500 µm.
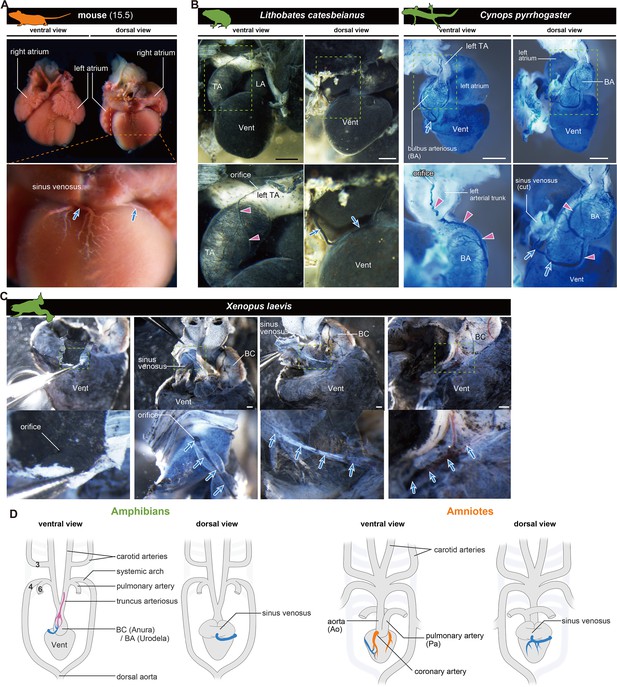
The orifices of the coronary veins are conserved among tetrapods.
Ink-injected hearts are shown. (A) Mouse. (B) American bullfrog (Lithobates catesbeianus) and the Japanese fire-belly newt (Cynops pyrrhogaster). (C) Xenopus laevis. Arteries (pink arrowheads) and veins (blue arrows) were found on the surface of the outflow tract. In (B) and (C), the coronary vein (blue arrows) branched from the sinus venosus and reached the outflow tract. (D) Summary. BA, bulbus arteriosus; BC, bulbus cordis; TA, truncus arteriosus; Vent, ventricle Scale bars: 1 mm.
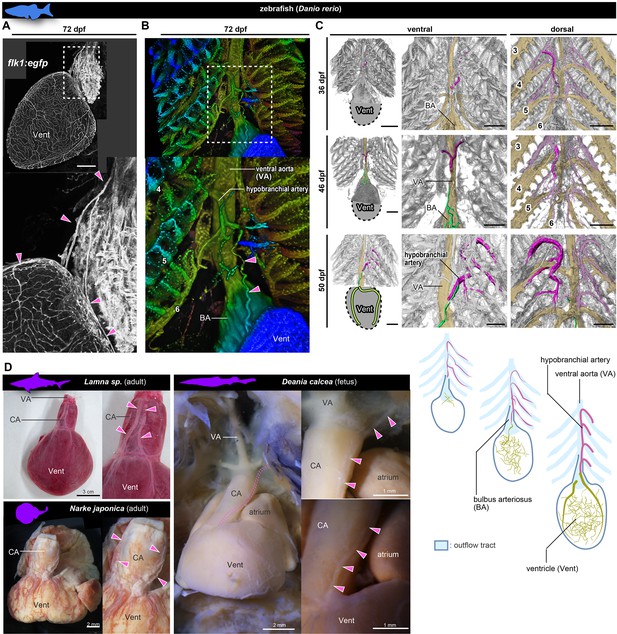
Anatomy and development of the coronary arteries of fishes.
(A, B) flk1:egfp zebrafish at 72 dpf (juvenile). (B) Three-dimensional image. (C) flk1:egfp zebrafish at 36, 46, and 50 dpf. The hypobranchial arteries (pink) arose from the dorsal side of the pharyngeal arch arteries. The vascular network (green) appeared on the ventricular surface at 46 dpf and connected with the hypobranchial artery at 50 dpf. The numbers indicate the branchial arteries. (D) Chondrichthyans. The anatomical pattern of the hypobranchial and coronary arteries was identical to that of zebrafish. BA, bulbus arteriosus; CA, conus arteriosus; VA, ventral aorta; Vent, ventricle. Scale bars: 100 µm.
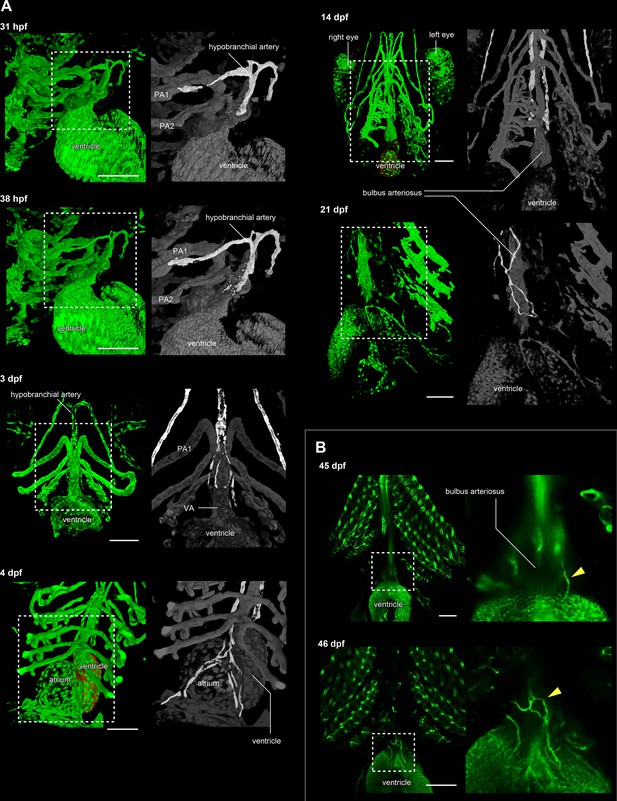
The development of hypobranchial artery in flk1:egfp zebrafish.
(A) Two-photon excitation microscopy images of the heart and aortic arch. The hypobranchial arteries are green in the magnified images (right column). At 3–4 dpf, an EGFP-positive endothelial bud appeared in the midline of the pharyngeal region and formed a Y-shaped structure. After 3 dpf, the hypobranchial artery expanded toward the cardiac outflow tract (ventral aorta). At 21 dpf, the hypobranchial artery formed a vascular plexus along the ventral aorta. (B) Confocal images of the ventral side of the heart. At 45 dpf, egfp-positive vessels formed a network on the surface of the outflow tract (bulbus arteriosus). At 46 dpf, these vessels became connected with the hypobranchial artery. PA1, first pharyngeal artery; PA2, second pharyngeal artery; VA, ventral aorta. Scale bars: 100 μm.
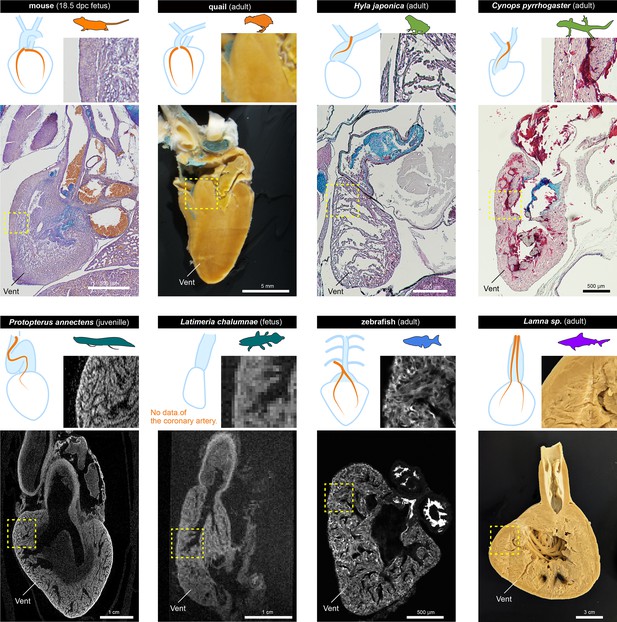
The histological structures of the ventricles.
The sagittal sections of the hearts of various jawed vertebrates. For the mouse, Hyla japonica, and Cynops pyrrhogaster histological sections stained with HE and Alcian blue are shown. For the lungfish (P. annectens) and coelacanth (L. chalumnae), micro CT and MRI scanned images are shown, respectively. Quail and shark (Lamna sp.) hearts were cut by hand. The distribution of coronary arteries is shown in the schemes. We could not observe any coronary arteries in the lungfish and coelacanths because of low resolution; the distribution of the arteries in the lungfish is based on previous reports (Szidon et al., 1969; Laurent et al., 1978). Vent, ventricle.
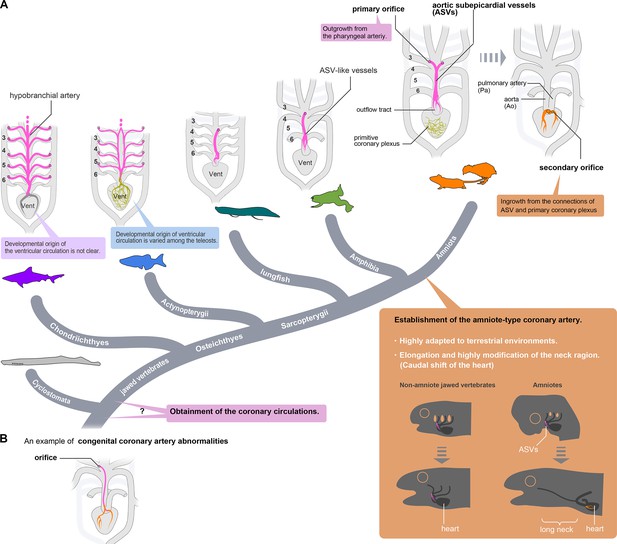
Evolution of coronary arteries.
(A) The ventricular coronary arteries unique to amniotes are reconstituted from the ASVs and the primitive coronary plexus. The ASV-like vessels of amphibians and the hypobranchial arteries of fishes are comparable to the ASVs, rather than to the ventricular coronary arteries of amniotes. It remains unclear whether the ventricular artery in chondrichthyans arises as an extension of the hypobranchial artery or has a different developmental origin. As ventricular arteries have a variety of developmental sources in teleosts, ancestral vessels in this region may have had a variety of developmental origins in different animal species. In amniotes, such redundant coronary arterial development gave way to a single robust and unchangeable developmental program, resulting in developmental constraints. (B) A scheme of congenital coronary artery abnormalities according to Kim et al., 2009.





