A back-door insight into the modulation of Src kinase activity by the polyamine spermidine
Figures
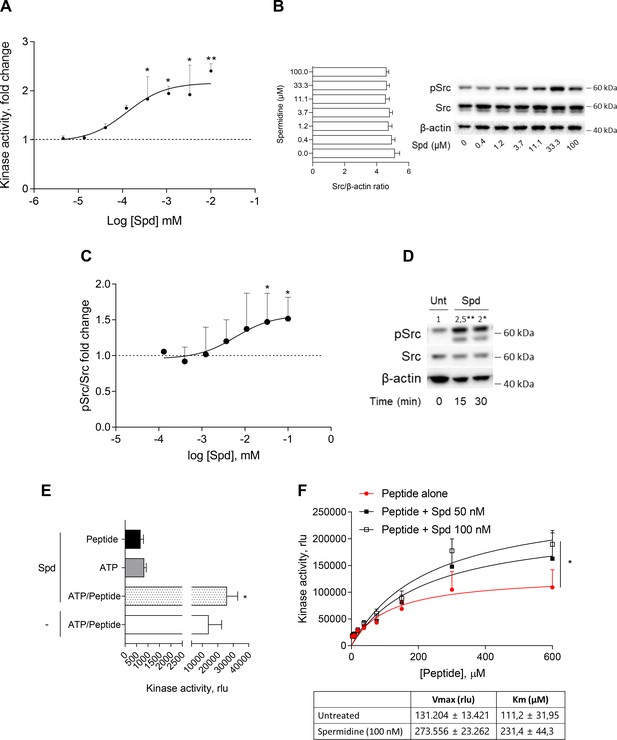
Spermidine enhances the activity of Src kinase in ATP-independent manner.
(A) Enzymatic activity of rhSrc in the presence of ATP (10 μM), synthetic peptide (100 μM), and increasing concentration of spermidine (45 nM to 100 μM). ADP-Glo Kinase Assay (Promega) was used to detect the activity. Results are shown as fold change vs untreated samples (fold change = 1, dotted line). Data are mean ± standard deviation (SD) of three independent experiments, each performed in triplicates. Data were analyzed with one-way analysis of variance (ANOVA) followed by post hoc Bonferroni test, by comparing the mean of spermidine-treated samples to untreated counterpart. *p < 0.05, **p < 0.01. Spermidine EC50 = 106.4 ± 13.4 nM. (B) Immunoblot analysis of phosphorylated (pSrc) and total Src protein level evaluated in cell lysates from SYF cells reconstituted with vector coding for wild-type Src and then treated with increasing concentration of spermidine (400 nM to100 μM). β-Actin expression was used as normalizer and the Src/β-actin ratio is included as mean ± SD of three independent experiments. One representative immunoblot of three is shown. (C) pSrc/Src ratio of scanning densitometry analysis of three independent immunoblots. Data (mean ± SD) are reported as fold change of samples treated with spermidine relative to untreated cells (fold change = 1, dotted line). Data were analyzed with one-way ANOVA followed by post hoc Bonferroni test, by comparing the mean of spermidine-treated samples to the untreated counterpart. *p < 0.05. Spermidine EC50 = 6.4 ± 0.6 μM. (D) Immunoblot analysis of phosphorylated (pSrc) and total Src protein level evaluated in cell lysates from MC38 cells treated with spermidine (20 μM) for the indicated time. β-Actin expression was used as normalizer. pSrc/Src ratio is calculated by densitometric quantification of the specific bands and is reported as fold change against untreated cells (fold change = 1). Data were analyzed with one-way ANOVA followed by post hoc Bonferroni test, by comparing the mean of spermidine-treated samples to the untreated counterpart. *p < 0.05, **p < 0.01 (E) Enzymatic activity of rhSrc in the presence of spermidine, with or without ATP and peptide substrate. Data are mean ± SD of three independent experiments and were analyzed by Student’s t-test comparing the Spd/ATP/peptide vs ATP/peptide sample. (F) Enzymatic activity of rhSrc in the presence of fixed concentrations of spermidine and increasing concentration of peptide substrate. Data are reported as mean ± SD of three independent experiments, each performed in triplicates. Vmax and Km were calculated after fitting the kinase activity data to the Michaelis–Menten equation. Data were analyzed with one-way ANOVA followed by post hoc Bonferroni test. *p < 0.05.
-
Figure 1—source data 1
Original immunoblots of phosphorylated (pSrc), total Src and actin protein levels evaluated in cell lysates from SYF cells reconstituted with vector coding for wild-type Src and then treated with increasing concentration of spermidine.
Figure with the uncropped blots with relevant bands clearly labeled are provided.
- https://cdn.elifesciences.org/articles/85872/elife-85872-fig1-data1-v2.zip
-
Figure 1—source data 2
Original immunoblots of phosphorylated (pSrc), total Src and actin protein levels evaluated in cell lysates from MC38 cells either treated with spermidine or left untreated for 15 and 30 min.
Figure with the uncropped blots with relevant bands clearly labeled are provided.
- https://cdn.elifesciences.org/articles/85872/elife-85872-fig1-data2-v2.zip
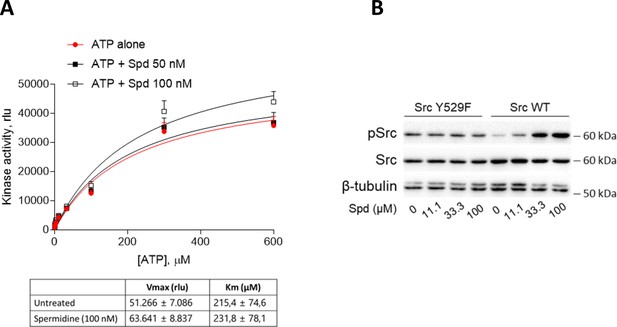
Spermidine does not compete with ATP and does not potentiate the constitutive active Src.
(A) Enzymatic activity of recombinant human Src in the presence of fixed concentration of spermidine and increasing concentration of ATP. Vmax and Km were calculated after fitting the kinase activity data to the Michaelis–Menten equation and reported in the table. Data are reported as mean ± standard deviation (SD) of three independent experiments, each performed in triplicates, and were analyzed with one-way analysis of variance (ANOVA) followed by post hoc Bonferroni test. (B) Immunoblot analysis of phosphorylated (pSrc) and total Src protein level in cell lysates from SYF cells either reconstituted with vector coding for wild-type Src (WT) or Src mutated at tyrosine 529 with phenilalanine (Y529F). Cells were then exposed to spermidine at the indicated concentrations. β-Tubulin expression was used as normalizer. One representative immunoblot of three is shown.
-
Figure 1—figure supplement 1—source data 1
Original immunoblots of phosphorylated, total Src and β-tubulin protein levels in lysates from SYF cells either reconstituted with vector coding for wild-type Src or Src mutated at tyrosine 529 with phenylalanine and then exposed to spermidine .
Figure with the uncropped blots with relevant bands clearly labeled are provided.
- https://cdn.elifesciences.org/articles/85872/elife-85872-fig1-figsupp1-data1-v2.zip
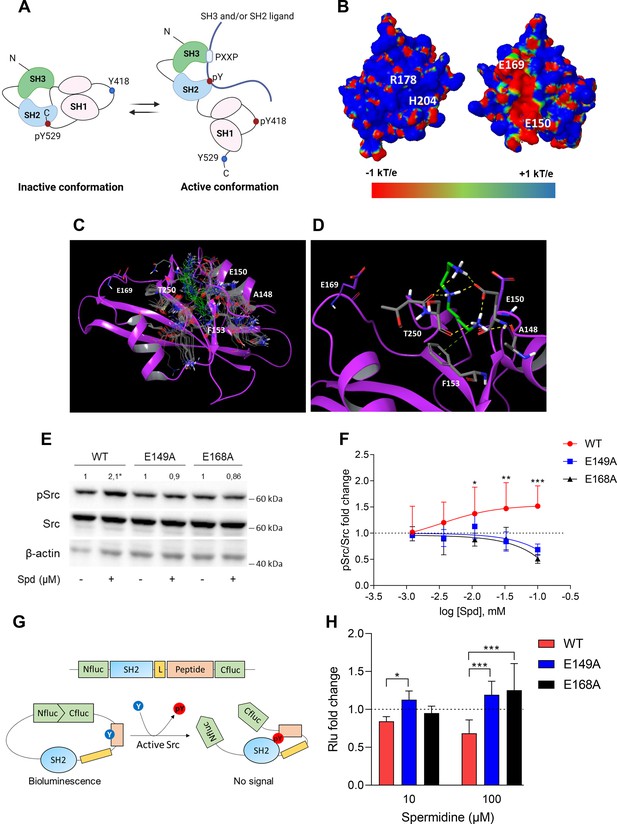
Spermidine binds to an allosteric site located in the SH2 domain of Src kinase.
(A) Schematic representation of the murine Src domains and kinase activation. The catalytic activation of the enzyme is characterized by the phosphorylation of the Y418 (pY418) in the activation loop. Created with BioRender.com. (B) Electrostatic potential surface of the Src SH2 domain showing the pY-binding site (R178 and H204) and the putative allosteric site for the endogenous polyamine as delimited by the glutamate residues (E150 and E169). Residues are labeled according to sequence numbering of the human isoform: R178, H204, E150, and E169 correspond to R32, H58, E4, and E23 of the NMR structure of the viral isoform, respectively. (C) Overlay of docking solutions of spermidine into the shallow cavity of Src kinase (poses #1–18, Supplementary file 1). Induced-fit conformations of side chains of residues shaping the cavity are shown with gray carbon-atoms according to each docking solution. Conformations of spermidine according to each docking solution are shown with green carbon-atoms. The Src SH2 domain is shown with magenta cartoon depicting the secondary structure. Residues are labeled according to sequence numbering of the human isoform. E150 and E169 residues are shown with magenta carbon-atoms. (D) Best energy-scored solution of the binding mode of spermidine into the allosteric pocket of Src (pose #1, Supplementary file 1). E149 and E168 are shown with magenta carbon-atoms. Interacting residues and spermidine are shown with gray and green carbon-atoms, respectively. Hydrogen bond interactions are shown with yellow dashed lines, while the π-cation interaction is reported with green dashed line. (E) Immunoblot analysis of phosphorylated (pSrc) and total Src protein level in cell lysates from SYF cells either reconstituted with vector coding for wild-type Src (WT) or Src mutated at glutamate 149 or 168 with alanine (E149A and E168A). Cells were then exposed to spermidine (100 μM). β-Actin expression was used as normalizer. pSrc/Src ratio is calculated by densitometric quantification of the specific bands and is reported as fold change against the corresponding untreated cells. Data were analyzed with one-way analysis of variance (ANOVA) followed by post hoc Bonferroni test, by comparing the mean of spermidine-treated samples to the untreated counterpart. *p < 0.05. (F) Activation of Src kinase in SYF cells treated with increasing concentration of spermidine (400 nM to 100 μM) and measured as pSrc/Src ratio of scanning densitometry analysis of three independent immunoblots. Results (mean ± standard deviation [SD]) are reported as fold change of samples treated with spermidine relative to untreated cells (fold change = 1, dotted line). (G) Schematic representation of the reporter functions. In the presence of active Src kinase, the phosphorylation of Src peptide results in its intramolecular interaction with the SH2 domain that prevents the complementation of split-luciferase fragments and generates a reduced bioluminescence activity. In the absence of Src activation, the N- and C-terminal luciferase domains are reconstituted and thus the bioluminescent activity is restored. (H) Measurement of luminescent signal in SYF cells co-expressing the reporter and the wild-type Src or its mutants (E149A and E168A), and then exposed to spermidine (10 and 100 µM). Results (mean ± SD of three independent experiments) are reported as fold change of bioluminescent signal in stimulated cells as compared to their respective untreated samples. Data (F, H) were analyzed with two-way ANOVA followed by post hoc Bonferroni test. *p < 0.05, **p < 0.01, ***p < 0.001.
-
Figure 2—source data 1
Original immunoblots of phosphorylated (pSrc), total Src and actin protein level evaluated in cell lysates from SYF cells either reconstituted with vector coding for wild-type Src (WT) or Src mutated at glutamate 149 or 168 with alanine.
Cells were either treated with spermidine (100 µM) or left untreated. Figure with the uncropped blots with relevant bands clearly labeled are provided.
- https://cdn.elifesciences.org/articles/85872/elife-85872-fig2-data1-v2.zip

The glutamate residues E149 and E168 are conserved across different species.
Alignment of the amino acid sequences of human, murine, and viral (2JYQ_1) Src. Conserved glutamate residues are highlighted in red.
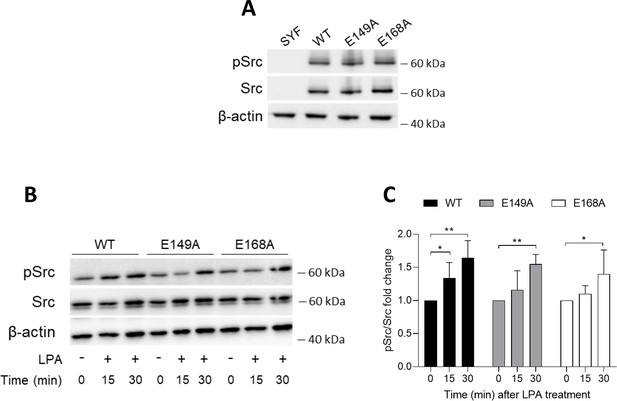
Efficient reconstitution of SYF cells with vectors coding for Src kinase.
(A) Immunoblot analysis of phosphorylated (pSrc) and total Src protein level in cell lysates from SYF cells either reconstituted with vector coding for wild-type Src (WT) or Src mutated at glutamate 149 or 168 with alanine (E149A and E168A). SYF cells transfected with empty vector (SYF) were used as control. β-Actin expression was used as normalizer. (B) Immunoblot analysis of phosphorylated (pSrc) and total Src protein level in cell lysates from SYF cells either reconstituted with vector coding for wild-type Src (WT) or Src mutated at glutamate 149 or 168 with alanine (E149A and E168A). Cells were then exposed to lysophosphatidic acid (LPA; 20 μM) for the indicated time. β-Actin expression was used as normalizer. One representative immunoblot of four is shown. (C) pSrc/Src ratio of scanning densitometry analysis of four independent experiments (mean ± standard deviation [SD]) expressed as fold change relative to the untreated counterparts (fold change = 1). Data were analyzed by one-way analysis of variance (ANOVA) followed by post hoc Bonferroni’s test. *p < 0.05, **p < 0.001.
-
Figure 2—figure supplement 2—source data 1
Original immunoblots of phosphorylated (pSrc), total Src and actin protein levels in cell lysates from SYF cells either reconstituted with vector coding for wild-type Src (WT) or Src mutated at glutamate 149 or 168 with alanine.
SYF cells transfected with empty vector (SYF) were used as control. Figure with the uncropped blots with relevant bands clearly labeled are provided.
- https://cdn.elifesciences.org/articles/85872/elife-85872-fig2-figsupp2-data1-v2.zip
-
Figure 2—figure supplement 2—source data 2
Original immunoblots of phosphorylated, total Src and actin protein levels in lysates from SYF cells either reconstituted with vector coding for wild-type Src or Src mutated at glutamate 149 or 168 with alanine.
Figure with the uncropped blots with relevant bands clearly labeled are provided.
- https://cdn.elifesciences.org/articles/85872/elife-85872-fig2-figsupp2-data2-v2.zip
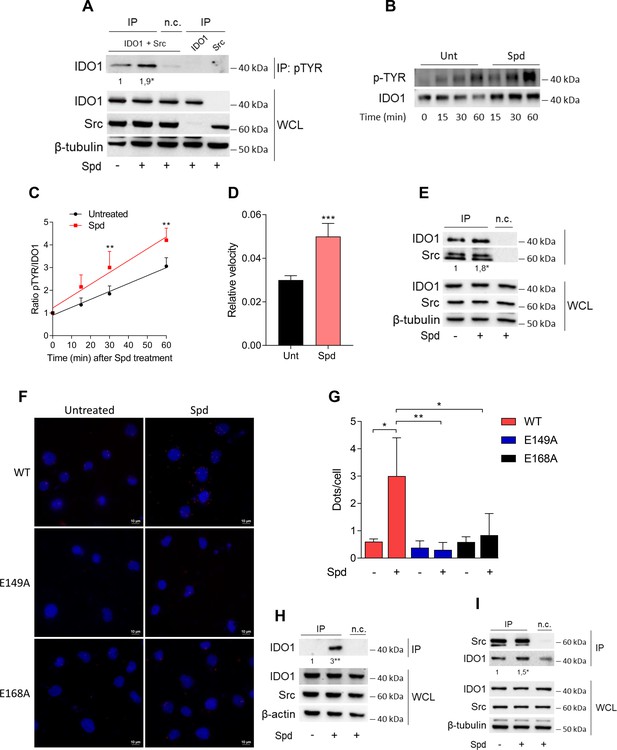
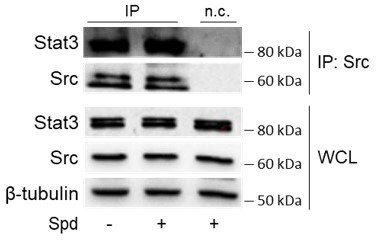
Spermidine triggers the phosphorylation of indoleamine 2,3-dioxygenase 1 (IDO1) by Src kinase and the complex formation.
(A) Immunoprecipitation with anti-phosphotyrosine antibody from SYF cells reconstituted with vectors coding for Src and IDO1 and then treated with spermidine (100 μM) for 60 min. Cells transfected with vectors coding for either Src or IDO1 were used as control, while immunoprecipitation without antibody was included as negative control (n.c.). The detection of IDO1, Src, and β-tubulin was performed by sequential immunoblotting with specific antibodies. Whole-cell lysates (WCL) was used as control of protein expression. One representative immunoblot of three is shown. The amount of IDO1 precipitated is measured by densitometric quantification of the specific bands in treated sample co-expressing IDO1 and Src and is reported relative to untreated cells (fold change = 1). Data (mean of three experiments) were analyzed with unpaired Student’s t-test. *p < 0.05. (B) Continuous in vitro kinase assay with rhIDO1 (300 ng) and rhSrc (50 ng) followed by immunoblot analysis with anti-phosphotyrosine and anti-IDO1 specific antibodies. The reaction was carried out for the indicated time, in either the presence or absence of spermidine. One representative immunoblot of three is shown. (C) pTYR/IDO1 signals were calculated by densitometric quantification of the specific bands. Data were plotted over incubation time of the kinase reaction and the slopes (relative velocity) of linear fits were calculated. Results (mean ± standard deviation [SD]) were analyzed with two-way analysis of variance (ANOVA) followed by post hoc Bonferroni test and by comparing, for each time point, the pTYR/IDO1 ratio of spermidine-treated sample to the untreated counterpart. (D) The relative velocity of the kinase reaction in either the presence or absence of spermidine from three independent experiments is shown. Data (mean ± SD) were analyzed with unpaired Student’s t-test. ***p < 0.001. (E) Immunoprecipitation of Src from SYF cells reconstituted with Src and IDO1, and then treated as in (A). The detection of IDO1, Src, and β-tubulin was performed by sequential immunoblotting with specific antibodies. Immunoprecipitation without antibody was included as negative control (n.c.). Whole-cell lysates (WCL) was used as control of protein expression. One representative immunoblot of three is shown. IDO1/Src ratio is calculated by densitometric quantification of the specific bands and is reported as fold change against untreated cells. Data (mean of three independent experiments) were analyzed with unpaired Student’s t-test. *p < 0.05. (F) The in situ proximity ligation assay between IDO1 and Src in SYF cells reconstituted with wild-type Src or the mutant forms and treated as in (A). Red spots indicate a single IDO1/Src interaction; scale bars, 10 µm. One representative experiment of three is shown. (G) Quantification of the interactions detected by proximity ligation assay using ImageJ. Results are reported as function of the number of cells. Data (mean ± SD) were analyzed with one-way ANOVA followed by post hoc Bonferroni test. *p < 0.05, **p < 0.01. (H) Immunoprecipitation with anti-phosphotyrosine antibody from MC38 cells either treated with spermidine (20 μM) for 60 min or left untreated. Immunoprecipitation without antibody was included as negative control (n.c.). The detection of IDO1, Src, and β-actin was performed by sequential immunoblotting with specific antibodies. Whole-cell lysates (WCL) was used as control of protein expression. One representative immunoblot of three is shown. The amount of IDO1 precipitated is measured by densitometric quantification of the specific band and is expressed relative to untreated cells (fold change = 1). Data (mean of three independent experiments) were analyzed with unpaired Student’s t-test. **p < 0.01. (I) Immunoprecipitation of Src from MC38 cells treated as in (H). The detection of IDO1, Src, and β-tubulin was performed by sequential immunoblotting with specific antibodies. Whole-cell lysates (WCL) was used as control of protein expression. One representative immunoblot of three is shown. IDO1/Src ratio is calculated by densitometric quantification of the specific bands and is reported as fold change against untreated cells (fold change = 1). Data (mean of three independent experiments) were analyzed with unpaired Student’s t-test. *p < 0.05.
-
Figure 3—source data 1
Original immunoblots of immunoprecipitation with anti-phosphotyrosine antibody (IP) followed by the detection of indoleamine 2,3-dioxygenase 1 (IDO1) with specific antibodies.
Whole-cell lysates (PRE-IP) was used as control of protein expression of IDO1, Src, and β-tubulin. SYF cells reconstituted with vectors coding for Src and IDO1 and then treated with spermidine (100 μM) for 60 min as well as cells transfected with vectors coding for either Src or IDO1 were used for the experiments. The negative control (i.e., the sample expressing both IDO1 and Src, but not immunoprecipitated with the antibody) is included. Figure with the uncropped blots with relevant bands clearly labeled are provided.
- https://cdn.elifesciences.org/articles/85872/elife-85872-fig3-data1-v2.zip
-
Figure 3—source data 2
Original immunoblots of in vitro kinase assay with rhIDO1 (300 ng) and rhSrc (50 ng) followed by immunoblot analysis with anti-phosphotyrosine and anti-IDO1 specific antibodies.
The reaction was in either the presence or absence of spermidine. Figure with the uncropped blots with relevant bands clearly labeled are provided.
- https://cdn.elifesciences.org/articles/85872/elife-85872-fig3-data2-v2.zip
-
Figure 3—source data 3
Original immunoblots of immunoprecipitation with anti-phosphotyrosine antibody (IP) followed by the detection of indoleamine 2,3-dioxygenase 1 (IDO1) with specific antibodies.
Whole-cell lysates (PRE-IP) of MC38 cells was used as control of protein expression of IDO1, Src, and β-actin. The negative control (i.e., sample not immunoprecipitated with the antibody) is included. Figure with the uncropped blots with relevant bands clearly labeled are provided.
- https://cdn.elifesciences.org/articles/85872/elife-85872-fig3-data3-v2.zip
-
Figure 3—source data 4
Original immunoblots of immunoprecipitation of Src from MC38 cells treated with spermidine or left untreated.
The detection of indoleamine 2,3-dioxygenase 1 (IDO1) and Src was performed by sequential immunoblotting with specific antibodies (IP). Whole-cell lysates (PRE-IP) was used as control of protein expression. The negative control (i.e., sample not immunoprecipitated with the antibody) is included. Figure with the uncropped blots with relevant bands clearly labeled are provided.
- https://cdn.elifesciences.org/articles/85872/elife-85872-fig3-data4-v2.zip
-
Figure 3—source data 5
Original immunoblots of immunoprecipitation of Src from MC38 cells treated with spermidine or left untreated.
The detection of indoleamine 2,3-dioxygenase 1 (IDO1) and Src was performed by sequential immunoblotting with specific antibodies (IP). Whole-cell lysates (PRE-IP) was used as control of protein expression. The negative control (i.e., sample not immunoprecipitated with the antibody) is included. Figure with the uncropped blots with relevant bands clearly labeled are provided.
- https://cdn.elifesciences.org/articles/85872/elife-85872-fig3-data5-v2.zip

Spermidine does not promote the phosphorylation of indoleamine 2,3-dioxygenase 1 (IDO1) via constitutive active Src.
Immunoprecipitation with anti-phosphotyrosine antibody from SYF cells reconstituted with vectors coding for Src (WT or Y529F) and IDO1, and then treated with spermidine (100 µM) for 60 min. Immunoprecipitation without antibody was included as negative control (n.c.). Cells transfected with vectors coding for either Src (WT or Y529F) or IDO1 were used as control. The detection of IDO1, Src, and β-tubulin was performed by sequential immunoblotting with specific antibodies. Whole-cell lysates (WCL) was used as control of protein expression. One representative immunoblot of three is shown. The amount of IDO1 immunoprecipitated is measured by densitometric quantification of the specific bands and is expressed relative to the respective untreated cells (fold change = 1). Data (mean of three experiments) were analyzed with one-way analysis of variance (ANOVA). *p < 0.05.
-
Figure 3—figure supplement 1—source data 1
Original immunoblots of immunoprecipitation with anti-phosphotyrosine antibody (IP) followed by the detection of indoleamine 2,3-dioxygenase 1 (IDO1) with specific antibodies.
Whole-cell lysates (PRE-IP) was used as control of protein expression of IDO1, Src, and β-tubulin. SYF cells were reconstituted with vectors coding for wild-type Src and IDO1 or Src mutated at tyrosine 529 with phenylalanine and IDO1. Moreover, cells transfected with vectors coding for either Src or IDO1 were used for the experiments. The negative control (i.e., sample expressing both IDO1 and Src, but not immunoprecipitated with the antibody) is included. Cells were either treated with spermidine (100 μM) or left untreated. Figure with the uncropped blots with relevant bands clearly labeled are provided.
- https://cdn.elifesciences.org/articles/85872/elife-85872-fig3-figsupp1-data1-v2.zip
Scheme of the Src kinase modulation by the polyamine spermidine.
Created with BioRender.com.
Tables
Primers for site-directed mutagenesis of Src.
| Primer | Sequence |
|---|---|
| Src E149A, Forward | atccaggctgaggcgtggtacttt |
| Src E149A, Reverse | aaagtaccacgcctcagcctggat |
| Src E168A, Forward | ctcaacgccgcgaacccgaga |
| Src E168A, Reverse | tctcgggttcgcggcgttgag |
| Src Y529F, Forward | gagccacagttccagcccgg |
| Src Y529F, Reverse | ccgggctggaactgtggctc |
Additional files
-
Supplementary file 1
Solutions of the docking study of spermidine into the allosteric site of Src SH2 domain using the structure of the viral isoform (PDB ID: 2JYQ).
- https://cdn.elifesciences.org/articles/85872/elife-85872-supp1-v2.docx
-
MDAR checklist
- https://cdn.elifesciences.org/articles/85872/elife-85872-mdarchecklist1-v2.pdf