A preclinical model of THC edibles that produces high-dose cannabimimetic responses
Figures
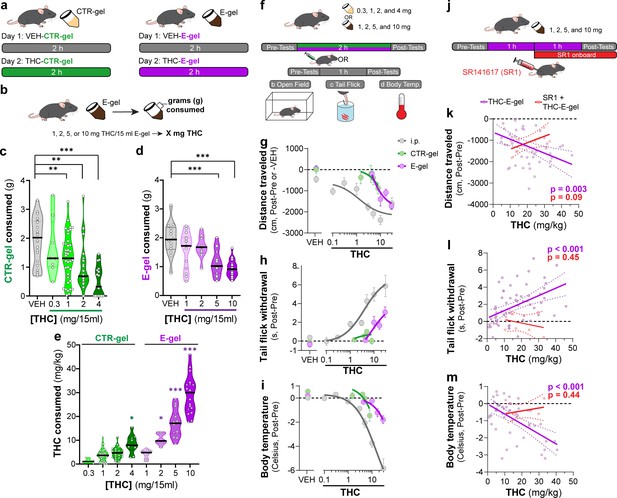
E-gel promotes heightened voluntary oral consumption of Δ9-tetrahydrocannabinol (THC) and induces cannabimimetic behaviors in adult mice.
(a) Mice were given free access to vehicle (VEH) or THC formulated in either CTR-gel or E-gel for 2 hr on days 1 and 2. (b) Consumption was determined by weighing gelatin at the end of each session. (c) Consumption of CTR-gel on day 2 is decreased after addition of THC. (d) Consumption of E-gel on day 2 is maintained after addition of THC. (e) Dose of THC consumed, in mg/kg, when formulated in either CTR-gel or E-gel on day 2. Results are mean ± SEM. Consumption compared ANOVA and Sidak’s, *p<0.05, **p<0.01, and ***p<0.001, N = 8–40. (f) Diagram of behavioral paradigm before and after intraperitoneal (i.p.) or gelatin administration. (g–i) Dose-dependent behavioral responses for hypolocomotion (g), analgesia (h), and hypothermia (i) after THC exposure. Administration by i.p. (gray) is plotted on x-axis by single bolus injection while CTR-gel (green) and E-gel (purple) are plotted based on average THC consumed after 2 hr exposure window shown in (e). (j) Diagram of THC-E-gel exposure, behavioral measurements, and SR1 injection (by i.p.) at 1 hr into exposure window. (k–m) Individual behavioral responses for hypolocomotion (k), analgesia (l), and hypothermia (m) for each animal. Individual points are plotted based on individual THC consumption with a linear regression to show correlation between consumed THC and behavioral output (p-values: k = 0.003, l < 0.001, m < 0.001). SR1-treated mice are plotted (red) based on consumed THC after exposure to 10 mg/15 ml THC-E-gel with a linear regression to show no correlation across three behaviors (p-values: k = 0.09, l = 0.44, m = 0.45).
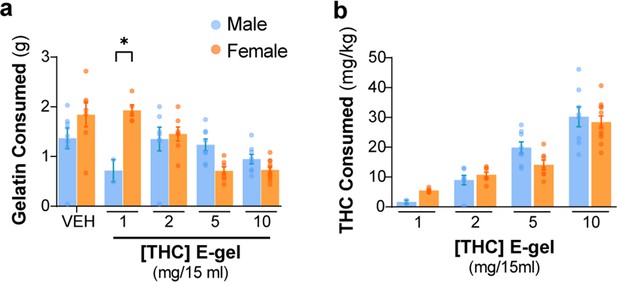
CTR-gel and E-gel consumption by male and female mice.
(a) Total E-gel consumed by males (blue) and females (orange) after 2 hr ad libitum access. (b) Calculated Δ9-tetrahydrocannabinol (THC) dose based on individual animal weights after E-gel consumption shown in (a). No statistical significance due to sex across doses was found from two-way ANOVA, Sidak’s post-hoc, N = 2–11. Results are mean ± SEM.
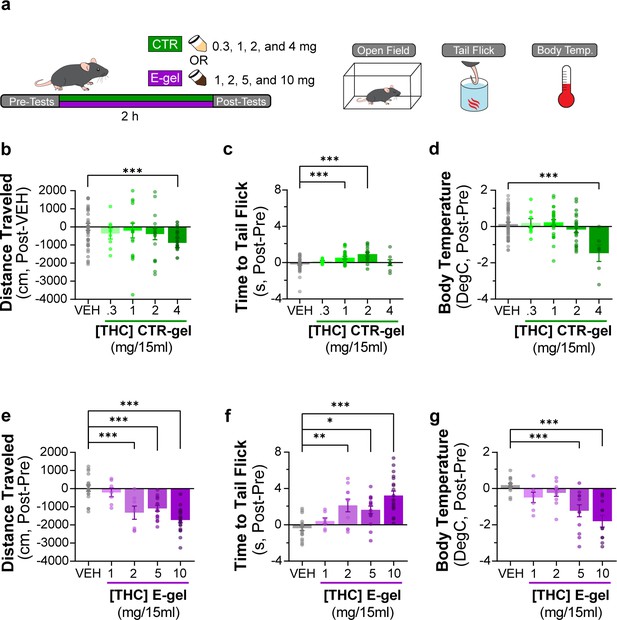
Triad behavioral responses after CTR-gel and E-gel consumption.
(a) Diagram outlining 2 hr exposure to either CTR-gel (green) or E-gel (purple) preceded and followed by triad behavioral tests. (b–d) Behavioral output immediately following CTR-gel administration for the triad of cannabimimetic behaviors measuring hypolocomotion (b), analgesia (c), and hypothermia (d). In (b), distance traveled for CTR-gel was calculated based on averaged vehicle (VEH) consumption. All errors expressed as SEM, unpaired Student’s t-test (***p<0.001). (e–g) Triad of cannabimimetic behaviors following Δ9-tetrahydrocannabinol (THC) E-gel administration measuring hypolocomotion (e), analgesia (f), and hypothermia (g). All error bars expressed as SEM, one-way ANOVA, Sidak’s post-hoc (*p<0.05, **p<0.01, ***p<0.001).
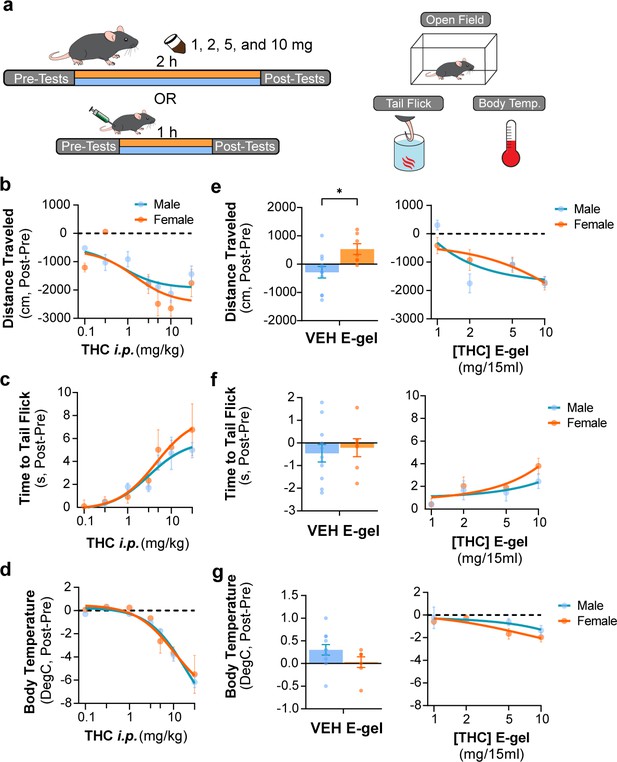
Triad behavioral responses to Δ9-tetrahydrocannabinol (THC) intraperitoneal (i.p.) and E-gel administration by male and female mice.
(a) Diagram outlining 2 hr exposure to either E-gel or i.p. for males (cyan) or females (orange) preceded and followed by triad behavioral tests. (b–d) Dose–response curves for the triad of hypolocomotion (b), analgesia (c), and hypothermia (d) cannabimimetic behaviors after i.p. THC administration with male responses shown in blue and female responses in orange. (e–g) Male and female behavioral responses after access to THC E-gel doses for all three triad cannabimimetic behaviors: hypolocomotion (e), analgesia (f), and hypothermia (g). Sex-specific responses to VEH E-gel are separated from concentration-dependent curves. All error bars expressed as SEM, two-way ANOVA, Sidak’s post-hoc (* p<0.05).
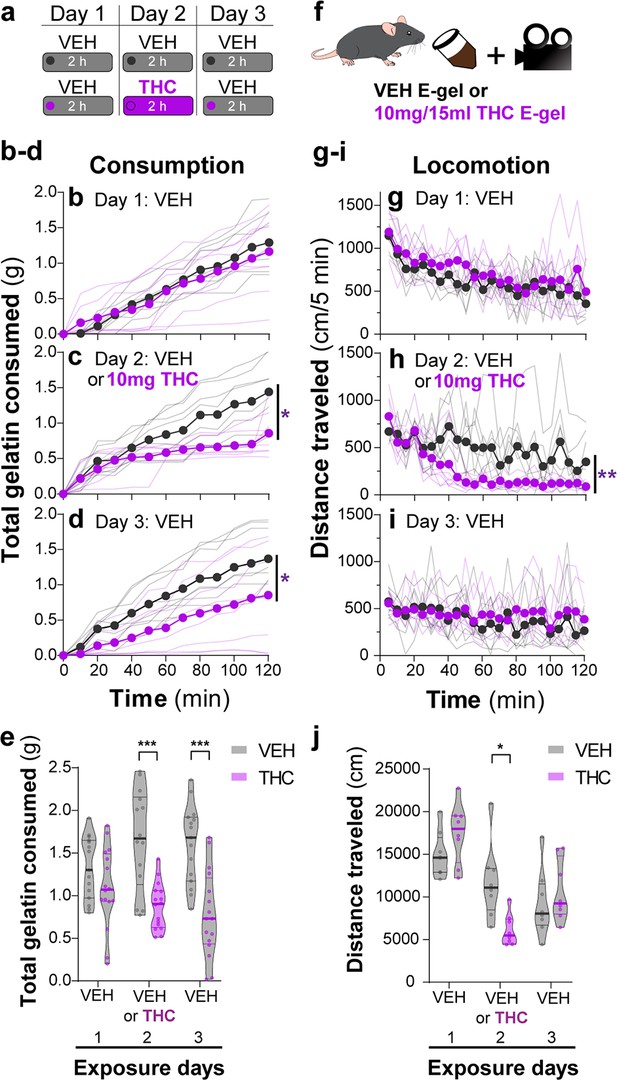
THC-E-gel consumption triggers CB1R-dependent behaviors.
(a) Over a 3-day exposure paradigm, mice received 3 d of E-gel with either vehicle (VEH) or Δ9-tetrahydrocannabinol (THC) (10 mg/15 ml) E-gel on day 2. (b–d) Cumulative gelatin consumption recorded every 10 min throughout the 2 hr exposure window over the 3-day paradigm. VEH (black) and THC (purple) groups received access to VEH on day 1 (b), VEH or THC on day 2 (c), and VEH on day 3 (d). (e) Total gelatin consumption after 2 hr of access to gelatin was plotted comparing VEH and THC treatment groups. (f) Animal consummatory and locomotor behavior was tracked during gelatin exposure window. (g–i) Distance traveled recorded every 5 min over the 3-day paradigm, similar to (b–d). (j) Total distance traveled (cm) after 2 hr of gelatin access was plotted comparing VEH and THC groups. Main effect over 2 hr exposure period (b–d, g–i) measured using two-way ANOVA with repeated measures and Sidak’s, main effect on total response (e, h) measured by one-way ANOVA and Sidak’s (*p<0.05, **p<0.01, ***p<0.001), N = 8–16.
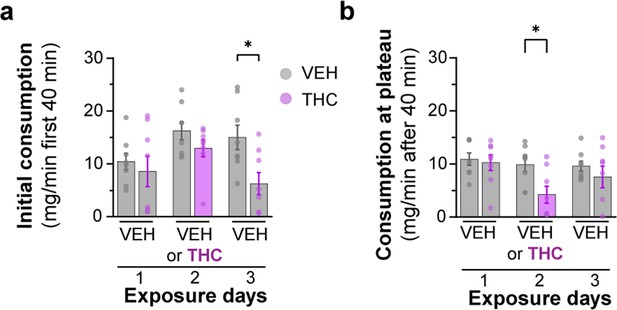
Analysis of Δ9-tetrahydrocannabinol (THC) E-gel consumption behavior over 3-day access paradigm.
(a) Rate of consumption during the first 40 min of 10 mg/15 ml THC E-gel access across the 3-day paradigm where the THC group received vehicle (VEH) E-gel on days 1 and 3. Error in SEM, unpaired Student’s t-test (*p<0.05). (b) Rate of consumption after the first 40 min of E-gel access just as in (a). Error in SEM, unpaired Student’s t-test (*p<0.05). (c) Latency to start consuming gelatin across the same experimental paradigm in (a) showing a statistical significance between VEH and THC groups but not within experimental days, two-way ANOVA, Sidak’s post-hoc, N = 8 (*p<0.05).
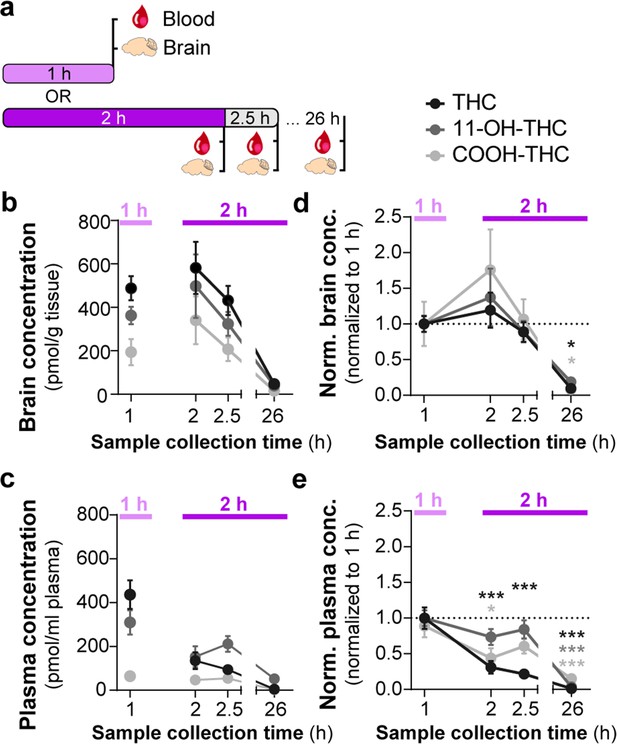
Consumption of Δ9-tetrahydrocannabinol (THC)-E-gel results in concomitant increases in the levels of THC and its metabolites in brain tissue.
(a) Diagram outlining gelatin exposure paradigm where blood and brain samples were collected immediately following 1 hr and at 2, 2.5, and 26 hr from the beginning of 2 hr access to 10 mg/15 ml THC-E-gel. (b) Brain concentration of THC, 11-OH-THC, and COOH-THC after E-gel exposure, 1 hr access is separated due to a reduced total access time to THC-E-gel compared to the other time points. (c) Plasma concentrations for the three compounds plotted similarly to (b). (d, e) PK concentrations in brain (d) and plasma (e) normalized to the 1 hr access period. Statistical comparison to 1 hr two-way ANOVA, Sidak’s, *p<0.05, **p<0.01, and ***p<0.001, N = 8–15.
-
Figure 3—source data 1
Brain tissue concentrations of Δ9-tetrahydrocannabinol (THC) and metabolites by sex.
- https://cdn.elifesciences.org/articles/89867/elife-89867-fig3-data1-v1.docx
-
Figure 3—source data 2
Plasma concentrations of Δ9-tetrahydrocannabinol (THC) and metabolites by sex.
- https://cdn.elifesciences.org/articles/89867/elife-89867-fig3-data2-v1.docx
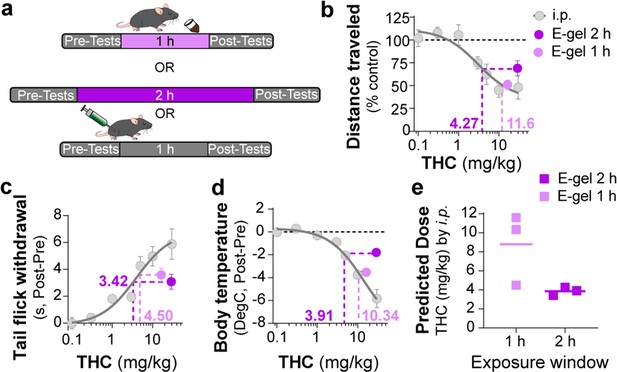
Correlating intraperitoneal (i.p.) Δ9-tetrahydrocannabinol (THC) and THC-E-gel triad cannabimimetic responses predicts THC-E-gel-dependent behaviors.
(a) Diagram of 1 hr and 2 hr THC-E-gel exposure and i.p. administration with behavioral tests. (b–d) Cannabimimetic responses after THC administration by i.p. and subsequent dose–response curve in gray. Responses after 1 hr or 2 hr exposure to 10 mg THC-E-gel are plotted with dotted lines tracking to relative THC-i.p. dose–response. (e) Predicted i.p. dose after 1 hr and 2 hr THC-E-gel exposure window from all three triad behaviors.
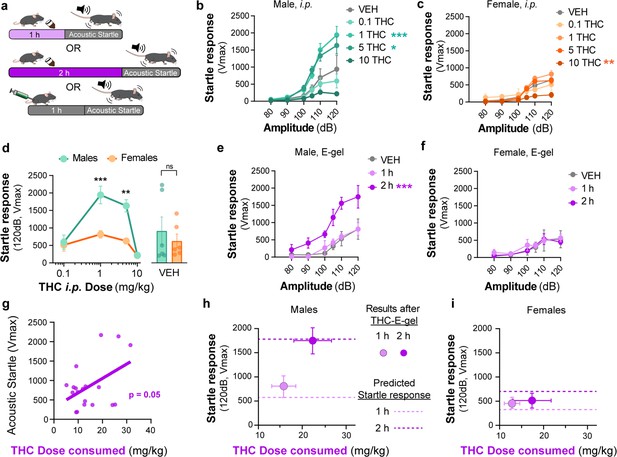
Sex-dependent acoustic startle responses after intraperitoneal (i.p.) injection of Δ9-tetrahydrocannabinol (THC) and high-concentration THC-E-gel consumption.
(a) Diagram of THC-E-gel exposure or i.p. administration followed by acoustic startle response behavioral testing. (b, c) Male and female acoustic startle responses after i.p. administration of THC in response to escalating tones (80, 90, 100, 105, 110, and 120 dB) following i.p. administration of THC in males (b) and females (c). (d) Male and female acoustic startle dose–responses to a 120 dB tone after i.p. THC administration. Results are mean ± SEM. one-way ANOVA, Sidak’s comparing vehicle (VEH) and i.p. THC dose between males and females, **p<0.01, ***p<0.001, N = 6–11. (e, f) Male and female acoustic startle responses after 1 hr or 2 hr THC E-gel exposure in response to escalating tones (80, 90, 100, 105, 110, and 120 dB). (g) THC dose consumption based on grams consumed and individual body weight correlated with individual acoustic startle response after 2 hr exposure. (h, i) Startle response to a 120 dB tone for males (h) and females (i) after 1 hr or 2 hr access to THC E-gel. Predicted doses calculated from a second-order polynomial of i.p. dose–responses are plotted to show the consistency in predicted dose–response after E-gel exposure.
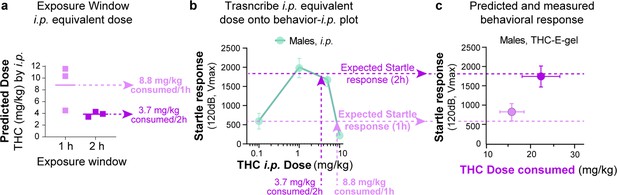
Methodology for Δ9-tetrahydrocannabinol (THC)--E-gel prediction of a behavioral response.
(a) Intraperitoneal (i.p.) equivalent dose calculated from the triad of cannabimimetic behaviors in Figure 4. (b) Transcribe i.p. equivalent doses to the THC-i.p. dose–response plot for the given behavior to find the predicted behavior after 1 hr or 2 hr exposure to 10 mg/15 ml THC-E-gel. (c) Predicted response (dashed line) and measured behavioral responses.





