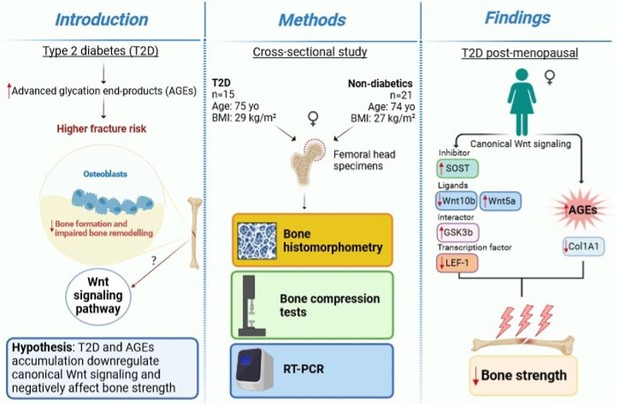
Bone canonical Wnt signaling is downregulated in type 2 diabetes and associates with higher advanced glycation end-products (AGEs) content and reduced bone strength
Figures
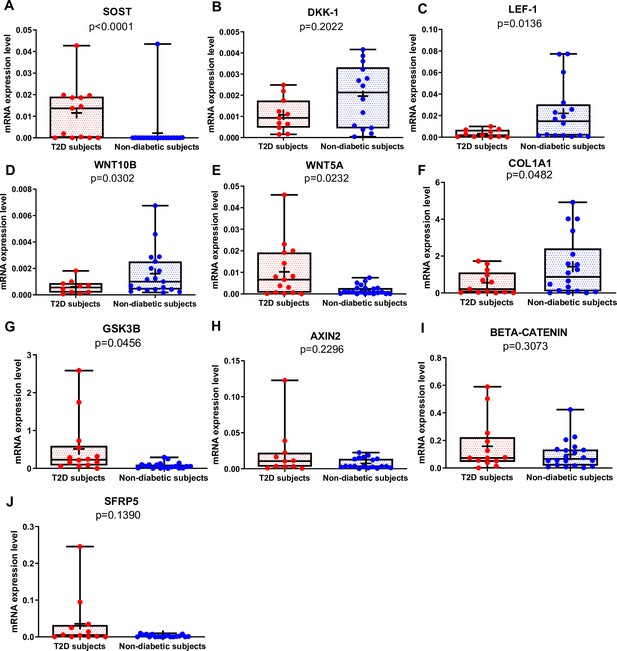
Gene expression analysis in trabecular bone samples.
(A) SOST mRNA levels resulted higher in type 2 diabetes (T2D) subjects versus non-diabetic subjects (p<0.0001). (B) DKK-1 mRNA expression level was not different between groups (p=0.2022). (C) LEF-1 mRNA levels resulted lower in T2D subjects versus non-diabetics subjects (p=0.0136). (D) WNT10B mRNA expression level was lower in T2D subjects versus non-diabetic subjects (p=0.0302). (E) WNT5A mRNA resulted higher in T2D subjects versus non-diabetics subjects (p=0.0232). (F) COL1A1 mRNA levels resulted lower in T2D subjects versus non-diabetic subjects (p=0.0482). (G) GSK3B mRNA levels resulted higher in T2D subjects versus non-diabetic subjects (p=0.0456). (H–J) AXIN2, BETA-CATENIN, SFRP5 mRNA levels were not different between groups (p=0.2296, p=0.3073, p=0.1390). Data are expressed as fold changes over beta-actin. Medians and interquartile ranges, differences between non-diabetics and T2D subjects were analyzed using Mann-Whitney test.
-
Figure 1—source data 1
Data represented by each point in Figure 1A–J.
- https://cdn.elifesciences.org/articles/90437/elife-90437-fig1-data1-v1.xlsx
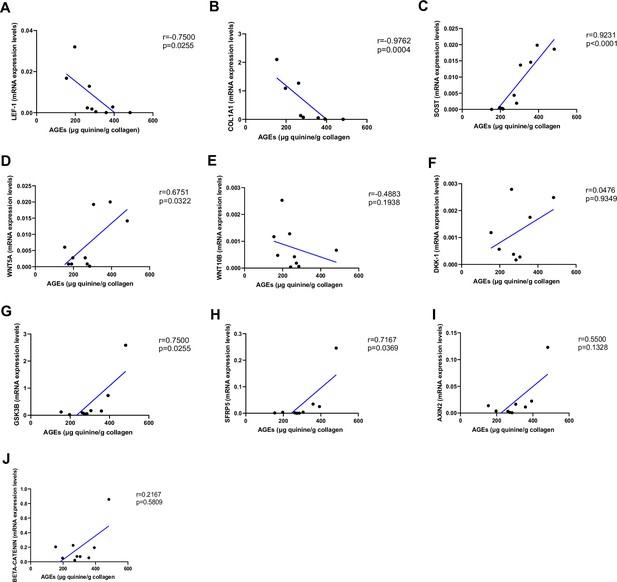
Relationship between advanced glycation end-products (AGEs) (µg quinine/g collagen) bone content and mRNA level of the Wnt signaling key genes in type 2 diabetes (T2D) and non-diabetic subjects.
(A) LEF-1 negatively correlated with AGEs (r=−0.7500; p=0.0255). (B) COL1A1 negatively correlated with AGEs (r=−0.9762; p=0.0004). (C) SOST mRNA level expression positively correlated with AGEs (r=0.9231; p<0.0001). (D) WNT5A mRNA expression level positively correlated with AGEs (r=0.6751; p=0.0322). (E) WNT10B mRNA expression level was not correlated with AGEs (r=−0.4883; p=0.1938). (F) DKK1 mRNA expression level was not correlated with AGEs (r=0.0476; p=0.9349). (G) GSK3B mRNA expression level was positively correlated with AGEs (r=0.7500; p=0.0255). (H) SFRP5 mRNA expression level was positively correlated with AGEs (r=0.7167; p=0.0369). (I) AXIN2 and (J) SFRP5 mRNA expression levels were not correlated with AGEs (r=0.5500, p=0.1328; r=0.2167, p=0.5809). Data were analyzed using nonparametric Spearman correlation analysis and r represents the correlation coefficient.
-
Figure 2—source data 1
Data represented by each point in Figure 2A–J.
- https://cdn.elifesciences.org/articles/90437/elife-90437-fig2-data1-v1.xlsx
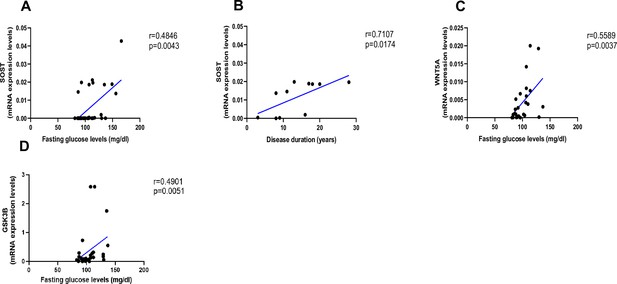
Relationship between fasting glucose levels (mg/dl) and disease duration with SOST and WNT5A mRNA levels.
(A) SOST positively correlated with fasting glucose levels (r=0.4846; p=0.0043). (B) SOST positively correlated with disease duration (r=0.7107; p=0.0174). (C) WNT5A positively correlated with fasting glucose levels (r=0.5589; p=0.0037). (D) GSK3B positively correlated with fasting glucose levels (r=0.4901; p=0.0051). Data were analyzed using nonparametric Spearman correlation analysis and r represents the correlation coefficient.
-
Figure 3—source data 1
Data represented by each point in Figure 3A–D.
- https://cdn.elifesciences.org/articles/90437/elife-90437-fig3-data1-v1.xlsx

Relationship between fasting glucose levels (mg/dl) and LEF 1, WNT5A, WNT10B, DKK-1, COL1A1 mRNA levels.
(A–E) Data showed negative correlations between fasting glucose levels (mg/dl) and (A) LEF-1 (r=–0.3649; p=0.0613), (B) WNT10B (r=–0.0041; p=0.9863), (C) COL1A1 (r=–0.1157; p=0.5354), (D) DKK-1 (r=–0.0947; p=0.6522) mRNA levels. Data showed positive correlations between fasting glucose levels (mg/dl) with (E) AXIN2 (r=0.0993; p=0.6442), (F) BETA-CATENIN (r=0.2371; p=0.1991), and (G) SFRP5 (r=0.3767; p=0.0696). Data were analyzed using nonparametric Spearman correlation analysis and r represents the correlation coefficient.
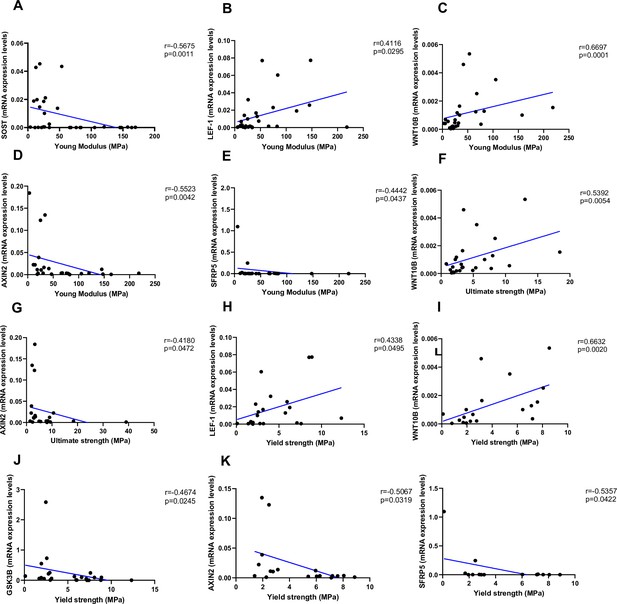
Relationship between Young’s modulus (MPa), ultimate strength (MPa), and yield strength (MPa) with mRNA levels of the Wnt signaling key genes in type 2 diabetes (T2D) and non-diabetic subjects.
(A) SOST negatively correlated with Young’s modulus (MPa); (r=−0.5675; p=0.0011). (B) LEF-1 positively correlated with Young’s modulus (MPa); (r=0.4116; p=0.0295). (C) WNT10B positively correlated with Young’s modulus (MPa); (r=0.6697; p=0.0001). (D) AXIN2 negatively correlated with Young’s modulus (MPa); (r=−0.5523; p=0.0042). (E) BETA-CATENIN negatively correlated with Young’s modulus (MPa); (r=−0.5244; p=0.0050). (F) SFRP5 negatively correlated with Young’s modulus (MPa); (r=−0.4442; p=0.0437). (G) WNT10B positively correlated with ultimate strength (MPa); (r=0.5392; p=0.0054). (H) AXIN2 negatively correlated with ultimate strength (MPa); (r=−0.4180; p=0.0472). (I) BETA-CATENIN negatively correlated with ultimate strength (MPa); (r=−0.5528; p=0.0034). (J) LEF-1 positively correlated with yield strength (MPa); (r=0.4338; p=0.0495). (K) WNT10B positively correlated with yield strength (MPa); (r=0.6632; p=0.0020). (L) GSK3B negatively correlated with yield strength (MPa); (r=−0.4674; p=0.0245). (M) AXIN2 negatively correlated with yield strength (MPa); (r=−0.5067; p=0.0319). (N) BETA-CATENIN negatively correlated with yield strength (MPa); (r=−0.5491; p=0.0149). (O) SFRP5 negatively correlated with yield strength (MPa); (r=−0.5357; p=0.0422). Data were analyzed using nonparametric Spearman correlation analysis and r represents the correlation coefficient.
-
Figure 4—source data 1
Data represented by each point in Figure 4A–L.
- https://cdn.elifesciences.org/articles/90437/elife-90437-fig4-data1-v1.xlsx
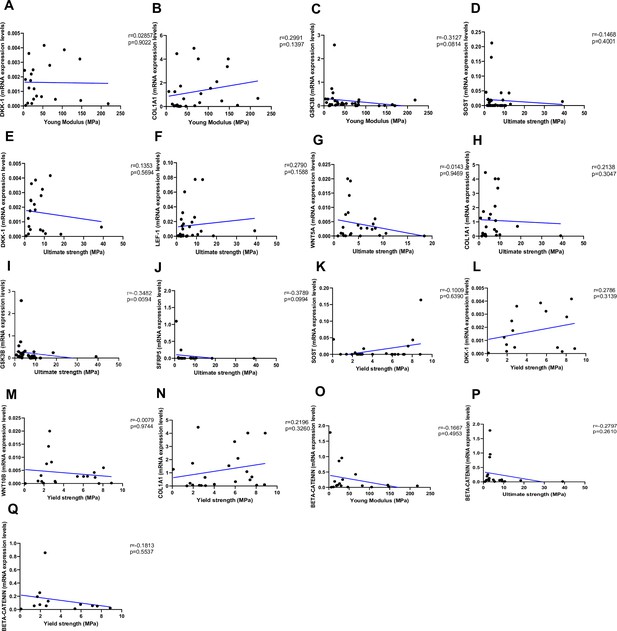
Relationship between Young’s modulus (MPa), ultimate strength (MPa), and yield strength (MPa) with mRNA levels of the Wnt signaling genes in type 2 diabetes (T2D) and non-diabetic subjects.
(A) DKK-1 positively correlated with Young’s modulus (MPa); (r=0.02857; p=0.9022). (B) COL1A1 positively correlated with Young’s modulus (MPa); (r=0.2991; p=0.1397). (C) GSK3B negatively correlated with Young’s modulus (MPa); (r=0.3127; p=0.0814). (D) SOST negatively correlated with ultimate strength (MPa); (r=-0.1468; p=0.4001). (E) DKK-1 negatively correlated with ultimate strength (MPa); (r=0.1353; p=0.5694). (F) LEF-1 positively correlated with ultimate strength (MPa); (r=0.2790; p=0.1588). (G) WNT5A negatively correlated with ultimate strength (MPa); (r=-0.0143; p=0.9469). (H) COL1A1 positively correlated with ultimate strength (MPa); (r=0.2138; p=0.3047). (I) GSK3B negatively correlated with ultimate strength (MPa); (r=-0.3482; p=0.0594). (J) SFPR5 negatively correlated with ultimate strength (MPa); (r=-0.3789; p=0.0994). (K) SOST positively correlated with yield strength (MPa); (r=0.1009; p=0.6390). (L) DKK-1 positively correlated with yield strength (MPa); (r=0.2786; p=0.3139). (M) WNT10B negatively correlated with yield strength (MPa); (r=–0.0079; p=0.9744). (N) COL1A1 positively correlated with yield strength (MPa); (r=0.2196; p=0.3260). (O) BETA-CATENIN negatively correlated with Young’s modulus strength (MPa); (r=–0.1667; p=0.4953). (P) BETA-CATENIN negatively correlated with ultimate strength (MPa); (r=–0.2797; p=0.2610). (Q) BETA-CATENIN negatively correlated with yield strength (MPa); (r=–0.1813; p=0.5537). Data were analyzed using nonparametric Spearman correlation analysis and r represents the correlation coefficient.
Tables
Clinical features of the study subjects.
Results were analyzed using unpaired t-test with Welch’s correction and are presented as median and percentiles (25th and 75th).
| T2D subjects(n=15) | Non-diabetic subjects(n=21) | p-Value | |||
|---|---|---|---|---|---|
| Age (years) | 73.00 (67.00–80.00) | 73.00 (68.50–79.00) | 0.644 | ||
| BMI (kg/m2) | 30.81 (24.44–34.00) | 25.00 (24.00–31.50) | 0.117 | ||
| Menopausal age (years) | 50.00 (42.50–52.75) | 52.00 (48.00–53.00) | 0.344 | ||
| Fasting glucose levels (mg/dl) | 112.00 (104.00–130.0) | 94.00 (87.25–106.3) | **0.009 | ||
| Disease duration (years) | 14.50 (7.25–19.25) | – | – | ||
| HbA1c (%) | 6.95 (6.37–7.37) | – | – | ||
| Serum calcium (mg/dl) | 9.05 (8.800–9.550) | 9.15 (9.000–9.550) | 0.535 | ||
| eGFR (ml/min/1.73 m2) | 78,30 (59.90–91.10) | 75.60 (61.35–88.55) | 0.356 | ||
| Serum blood urea nitrogen (mg/dl) | 42.00 (36.00–53.00) | 37.00 (31.75–46.50) | 0.235 | ||
-
** p value ≤ 0.01.
Histomorphometric parameters of trabecular bone of the study subjects.
Results were analyzed using unpaired t-test with Welch’s correction and are presented as median and percentiles (25th and 75th).
| T2D subjects(n=9) | Non-diabetic subjects(n=9) | p-Value | |
|---|---|---|---|
| BV/TV (%) | 0.248 (0.157–0.407) | 0.358 (0.271–0.456) | 0.120 |
| Md.V/BV (%) | 0.994 (0.984–0.998) | 0.995 (0.985–0.997) | 0.998 |
| Md.V/TV (%) | 0.249 (0.156–0.366) | 0.352 (0.269–0.454) | 0.053 |
| OV/BV (%) | 0.009 (0.002–0.009) | 0.004 (0.002–0.015) | 0.704 |
| OV/TV (%) | 0.001 (0.0002–0.0058) | 0.001 (0.0007–0.0056) | 0.896 |
| OS/BS (%) | 0.026 (0.022–0.161) | 0.035 (0.009–0.117) | 0.525 |
Bone mechanical parameters of trabecular bone of the study subjects.
Results were analyzed using unpaired t-test with Welch’s correction and are presented as median and percentiles (25th and 75th).
| T2D subjects(n=11) | Non-diabetic subjects(n=21) | p-Value | |
|---|---|---|---|
| Young’s modulus (MPa) | 21.60 (13.46–30.10) | 76.24 (26.81–132.9) | 0.002 |
| Ultimate strength (MPa) | 3.015 (2.150–13.86) | 7.240 (3.150–8.898) | 0.914 |
| Yield strength (MPa) | 2.525 (1.943–6.393) | 6.150 (3.115–7.423) | 0.159 |