Chemotherapy activates inflammasomes to cause inflammation-associated bone loss
Abstract
Chemotherapy is a widely used treatment for a variety of solid and hematological malignancies. Despite its success in improving the survival rate of cancer patients, chemotherapy causes significant toxicity to multiple organs, including the skeleton, but the underlying mechanisms have yet to be elucidated. Using tumor-free mouse models, which are commonly used to assess direct off-target effects of anti-neoplastic therapies, we found that doxorubicin caused massive bone loss in wild-type mice, a phenotype associated with increased number of osteoclasts, leukopenia, elevated serum levels of danger-associated molecular patterns (DAMPs; e.g. cell-free DNA and ATP) and cytokines (e.g. IL-1β and IL-18). Accordingly, doxorubicin activated the absent in melanoma (AIM2) and NLR family pyrin domain containing 3 (NLRP3) inflammasomes in macrophages and neutrophils, causing inflammatory cell death pyroptosis and NETosis, which correlated with its leukopenic effects. Moreover, the effects of this chemotherapeutic agent on cytokine secretion, cell demise, and bone loss were attenuated to various extent in conditions of AIM2 and/or NLRP3 insufficiency. Thus, we found that inflammasomes are key players in bone loss caused by doxorubicin, a finding that may inspire the development of a tailored adjuvant therapy that preserves the quality of this tissue in patients treated with this class of drugs.
eLife assessment
This useful study, which systematically addresses off-target effects of a commonly used chemotherapy drug on bone and bone marrow cells and which therefore is of potential interest to a broad readership, presents evidence that reducing systemic inflammation induced by doxorubicin limits bone loss to some extent. The demonstration of the effect of systemic inflammation on bone loss is convincing. Building on prior work, this study sets the scene for additional genetic and pharmacologic experiments as well as future analyses of the bone phenotypes, which should speak to the mechanisms involved in doxorubicin-induced bone loss – which are not addressed in the current study – and which may substantiate the clinical relevance of targeting inflammation in order to limit the negative impact of chemotherapies on bone quality.
https://doi.org/10.7554/eLife.92885.4.sa0Introduction
The chemotherapeutic drug, doxorubicin, is widely used for the treatment of breast cancer, bladder cancer, lymphomas, and acute lymphocytic leukemia (Smith et al., 2010; Jang et al., 2023; Geffen and Man, 2002). Despite its success in improving the survival rate of cancer patients, doxorubicin causes serious adverse effects, including cardiomyopathy, bone marrow suppression, hair loss, and skeletal manifestations (Cardinale et al., 2015; Tacar et al., 2013; Coleman et al., 2013). Bone complications include osteoporosis, a metabolic disease that is characterized by decreased bone mass and deteriorated microarchitecture, and associated with increased risks for the development of late fractures, and morbidities in the elderly populations (Cauley and Lui, 2009; Cawthon et al., 2009; Cawthon et al., 2012). In fact, it was reported that 20–50% of geriatric patients (≥65 years) with a hip fracture die within 1 year of fracture (Coleman et al., 2013). Consistent with the dogma that bone resorption by osteoclasts (OCs) and bone formation by osteoblasts is uncoupled in osteoporotic patients, doxorubicin causes bone loss by promoting osteoclastogenesis while suppressing osteoblastogenesis (Yao et al., 2020; Chai et al., 2014; Rana et al., 2013; Zhou and Kuai, 2020). Increased production of senescence-associated secretory phenotype, enhanced generation of reactive oxygen species, and dysregulated autophagy and mitochondrial metabolism are proposed mechanisms of doxorubicin-induced bone pathology (Yao et al., 2020; Park et al., 2022).
Doxorubicin intercalates into DNA thereby impeding the activity of DNA repair enzymes such as topoisomerase II and impairing DNA replication (Robson et al., 1987; Lawrence, 1988; Bonner and Lawrence, 1990; Abe et al., 2022; Tewey et al., 1984). Defective DNA repair ultimately culminates in genomic instability and cell demise, events that can provoke uncontrollable release of intracellular contents such as DNA and various danger-associated molecular patterns (DAMPs). DNA-enriched entities include neutrophil extracellular traps (NETs), web-like structures in which DNA is decorated with peptides, some of which have anti-microbial and inflammatory properties (Komada et al., 2018; Apel et al., 2021). NETs can also propagate inflammation following their engulfment by phagocytes (Boccia et al., 2022; Blayney and Schwartzberg, 2022; Nakazawa et al., 2016; Jeong et al., 2021). Since DNA normally resides in the nucleus and mitochondria, its presence in the cytoplasm is detected by DNA sensors, including absent in melanoma 2 (AIM2), and cyclic guanosine monophosphate-adenosine monophosphate synthase, which can trigger immune responses aimed at eliminating the mislocated DNA (Sun et al., 2013; Zhang et al., 2014; Rathinam et al., 2010). Oxidized DNA and various DAMPs can also be sensed by NLRP3 (Shimada et al., 2012; Lu et al., 2014; Xian et al., 2022). Upon recognition of DAMPs or pathogen-associated molecular patterns (PAMPs), AIM2 and NLRP3 assemble protein platforms comprising the adaptor protein apoptosis-associated speck-like protein containing a CARD (ASC) and caspase-1. These protein complexes known as inflammasomes are responsible for the maturation of pro-interleukin-1β (pro-IL-1β) and pro-IL-18 to IL-1β and IL-18, respectively (Guo et al., 2015; Sharma and Kanneganti, 2021; Wang et al., 2021). Inflammasome-comprising caspase-1 also cleaves gasdermin D (GSDMD), generating N-terminal fragments, which form IL-1β- and IL-18-secreting conduits, and cause the inflammatory cell death, pyroptosis (Guo et al., 2015; Sharma and Kanneganti, 2021; Wang et al., 2021). While acute activation of inflammasomes is important for the clearance of the perceived danger and restoration of homeostasis, chronic or excessive stimulation of these safeguard mechanisms can cause disease.
Inflammasomes are involved in skeletal pathophysiology. Gain-of-function mutations of NLRP3 cause skeletal abnormalities in humans (Aksentijevich et al., 2002; Feldmann et al., 2002), which are reproduced to a great extent in knock-in mice expressing NLRP3 harboring mutations found in these patients (Bonar et al., 2012; Qu et al., 2015; Snouwaert et al., 2016; Wang et al., 2017). In normal mice, degraded bone matrix components, which are released during bone resorption, promote inflammasome activation and OC differentiation (Alippe et al., 2017). Age-associated bone loss has also been linked to chronic low-grade inflammation mediated by the NLRP3 inflammasome (Youm et al., 2013). More relevant to this study, radiation, which is also used as an anti-neoplastic therapy, causes bone loss through GSDMD downstream of AIM2 and NLRP3 inflammasomes, but not NLR family caspase recruitment domain containing protein 4 (NLRC4) inflammasome (Xiao et al., 2020). Collectively, the bone phenotypes of genetically or pharmacologically activated inflammasome sensors suggest that the fate of bone cells can be influenced by inflammation driven by inflammasomes, which are mainly activated in myeloid cells (Brydges et al., 2009; Meng et al., 2009). This view provides a strong rationale for exploring the role of inflammasome pathways in bone loss induced by off-target actions of doxorubicin as this agent causes the death of cancer and bystander normal cells, thereby releasing DAMPs such as ATP and DNA, which activate these pathways.
We used non-tumor-bearing mouse models, which are commonly used to assess off-target outcomes of anti-neoplastic therapies (Park et al., 2022; Borniger et al., 2015; Harrison et al., 1980; Yao et al., 2020) to study bone adverse effects of doxorubicin. We found that doxorubicin caused massive bone loss in wild-type (WT) mice, a phenotype associated with increased number of OCs, leukopenia, and cytokinemia. These outcomes implicated the AIM2 and NLRP3 inflammasomes as they were attenuated upon genetic inactivation of these sensors. Thus, our results show that inflammasomes are key players in bone loss caused by doxorubicin, a finding that may enable the implementation of novel strategies for chemotherapy-related bone complications.
Results
Doxorubicin causes bone loss
To determine the effects of doxorubicin on bone mass, femurs of 10-week-old WT female mice were analyzed by VivaCT before (baseline) and 4 weeks after a single intraperitoneal injection of 5 mg/kg doxorubicin or vehicle. Doxorubicin, but not the vehicle, caused bone loss (Figure 1—figure supplement 1A–D). Doxorubicin also caused bone loss in 10-week-old WT male mice, a response that was associated with increased OC number and surface (Figure 1A–E) and decreased bone formation (Figure 1—figure supplement 1E). These findings were consistent with the recently reported stimulatory and suppressive effects of this drug on bone resorption and formation in WT female mice, respectively (Yao et al., 2020). Since doxorubicin inflicted bone damage independently of the sex, afterward mechanistic studies focused mainly on male mice and revolved around innate immune responses, which regulate OC-mediated bone resorption in pathological conditions.
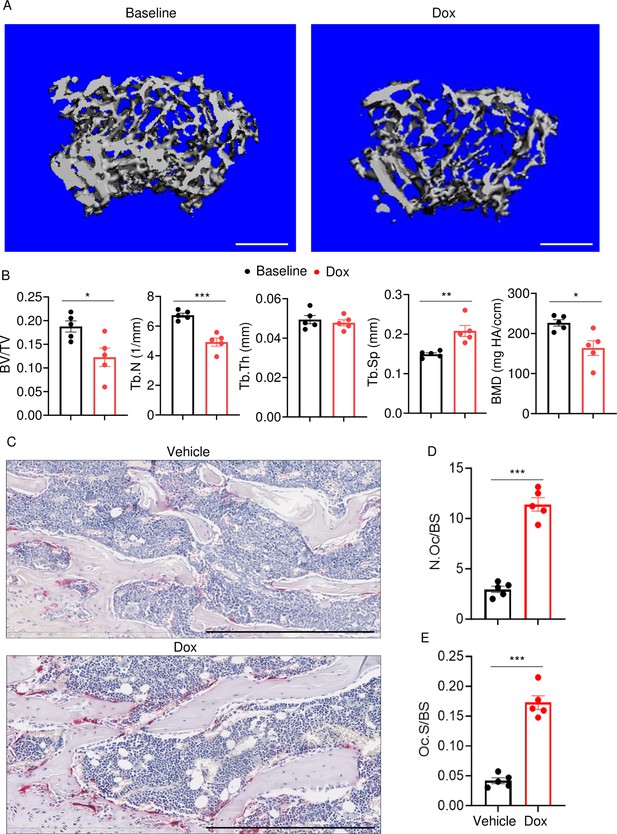
Doxorubicin causes bone loss in male mice.
Femurs from WT male mice were analyzed by VivaCT before (baseline) and 4 weeks after a single intraperitoneal injection of 5 mg/kg doxorubicin. Cross sections of 3D reconstructions. Scale bars: 500 μm (A), bone parameters (B), and femurs from WT male mice (C–E) were analyzed 4 weeks after a single intraperitoneal injection of vehicle or doxorubicin. Specimens were stained for tartrate-resistant acidic phosphatase (TRAP) activity. Representative images. Scale bars: 500 μm (C), N.Oc/BS (D), Oc.S/BS (E). N=5 mice/group. Data are mean ± SEM. Student’s t-test was used. *p<0.05; **p<0.01; ***p<0.001. BMD, bone mineral density; BV/TV, bone volume/total volume; Dox, doxorubicin; N.Oc/BS, OC number/bone surface; Oc.S/BS, OC surface/bone surface; OC, osteoclast; ns, not significant; Tb.N, trabecular number; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; WT, wild-type.
-
Figure 1—source data 1
Micro-computed tomography (µCT) analysis in Figure 1B.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig1-data1-v1.xlsx
-
Figure 1—source data 2
Tartrate-resistant acidic phosphatase (TRAP) staining analysis in Figure 1D and E.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig1-data2-v1.xlsx
Doxorubicin causes cytokinemia, leukopenia, release of DAMPs, and NETosis in vivo
Doxorubicin induces inflammatory responses in patients and experimental models (Wittenburg et al., 2019). Accordingly, WT mice exposed to doxorubicin for 3 days exhibited higher serum levels of IL-1β, IL-18, IL-6, and TNF-α compared to vehicle-injected counterparts (Figure 2A–D). Levels of these inflammatory cytokines inversely correlated with the abundance of white blood cells (WBCs; Figure 2E). While doxorubicin lowered the number of circulating lymphocytes and monocytes, the number of neutrophils, the most abundant immune cells in the blood, increased 2 hr post-drug exposure before progressively returning to baseline levels. Consistent with the leukopenic outcome, levels of ATP, which is released by dead cells, were higher in doxorubicin-treated mice compared to vehicle-treated cohorts (Figure 2F). To gain further insight into the mechanisms of leukopenia, we focused on neutrophils, the most abundant leukocytes in blood. These cells exhibit morphological changes such as NET extrusion upon exposure to PAMPs or sterile DAMPs, and eventually undergo NETosis (Komada et al., 2018; Apel et al., 2021). To determine the effects of doxorubicin on NET formation, we measured serum levels of NET components in mice treated with vehicle or this drug for 48 hr. We found that doxorubicin induced NET formation as evidenced by increased levels of citrullinated histone 3 (Cit-H3; Figure 2G), myeloperoxidase (MPO; Figure 2H), and cell-free DNA (cfDNA) (Figure 2I and J). Thus, doxorubicin causes cytokinemia, a response that is associated with increased cell death and decreased number of WBCs.
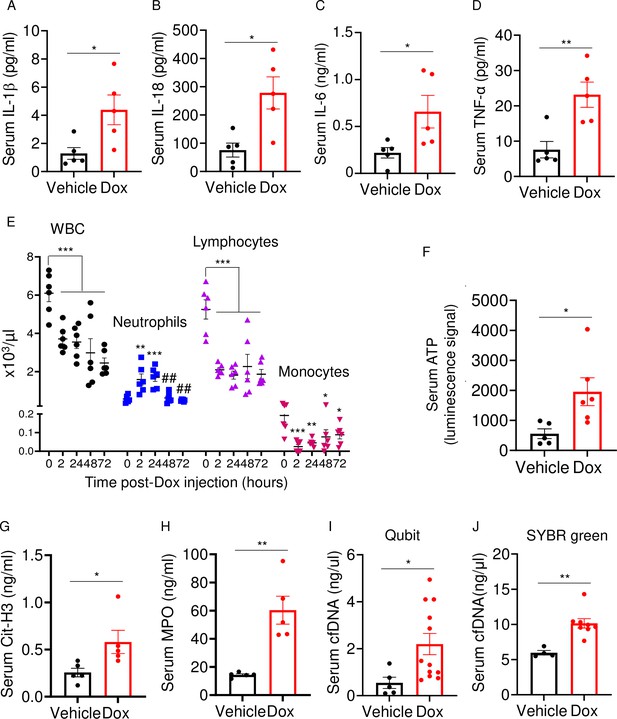
Doxorubicin causes cytokinemia, leukopenia, release of danger-associated molecular patterns (DAMPs), and NETosis in vivo.
Twelve-week-old WT mice were exposed to a single dose of vehicle or 5 mg/kg doxorubicin. Serum samples were harvested 3 days (A–D) or 2 days (F–J) later and analyzed by MSD (IL-1β, IL-6, and TNF-α) or ELISA (Cit-H3, IL-18, and MPO). Blood was collected for cell counts at the indicated time-points after a single dose of 5 mg/kg doxorubicin injection (E). cfDNA was measured using Qubit (I) or SYBR green (J). Data are mean ± SEM. N=5–12 mice/group. *p<0.05; **p<0.01; ***p<0.001 vs 0 hr; ##p<0.01 vs 2 or 24 hr. Student’s t-test (A–D, F–J) and one-way ANOVA (E) were used. cfDNA, cell-free DNA; Cit-H3, citrullinated histone H3; Dox, doxorubicin; IL, interleukin; MPO, myeloperoxidase; WBCs, white blood cells.
-
Figure 2—source data 1
Serum samples analysis of inflammatory cytokines, ATP, citrullinated histone 3 (Cit-H3), myeloperoxidase (MPO), and cfDNA in Figure 2A–D and F–J.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig2-data1-v1.xlsx
-
Figure 2—source data 2
Complete blood count in Figure 2E.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig2-data2-v1.xlsx
Doxorubicin activates inflammasome-dependent and -independent pathways, and causes macrophage pyroptosis
The effects of doxorubicin on macrophages have been reported (Chen et al., 2023; Saleh et al., 2021). To test the hypothesis that these cells were implicated in the inflammatory phenotype of mice treated with doxorubicin, we treated bone marrow-derived macrophages (BMMs) with lipopolysaccharide (LPS) for 3 hr to induce the expression of inflammasome components (Wang et al., 2021), then with various concentrations of this drug for 16 hr. Within its reported potent concentrations (1.5–12 µM) (Saleh et al., 2021; Eljack et al., 2022), doxorubicin did not induce IL-1β secretion, but it significantly caused the release of lactate dehydrogenase (LDH; Figure 3A and B), a marker of cell death as it is released only upon plasma membrane rupture (Wang et al., 2021). Since LDH release was not induced by doxorubicin in a dose-dependent manner, this response may be the result of non-selective cytotoxic actions of this drug. By contrast, doxorubicin promoted IL-1β and LDH release by LPS-primed BMMs in a dose-dependent fashion, with the maximal effect on IL-1β secretion achieved at 6 µM (Figure 3A). Unexpectedly, LPS attenuated LDH release induced by low doxorubicin concentrations (1. 5 and 3 µM) (Figure 3B). To further gain insight into the mechanism of action of doxorubicin, we measured the levels of ATP, which is released by dead cells and activates multiple pathways, including the NLRP3 inflammasome (Karmakar et al., 2016; Carta et al., 2015). ATP levels were higher in the supernatants of doxorubicin-treated BMMs compared to controls, a response that was further enhanced by LPS (Figure 3C). Collectively, these results suggest that BMMs underwent pyroptosis in the presence of LPS and doxorubicin, releasing DAMPs such as ATP.
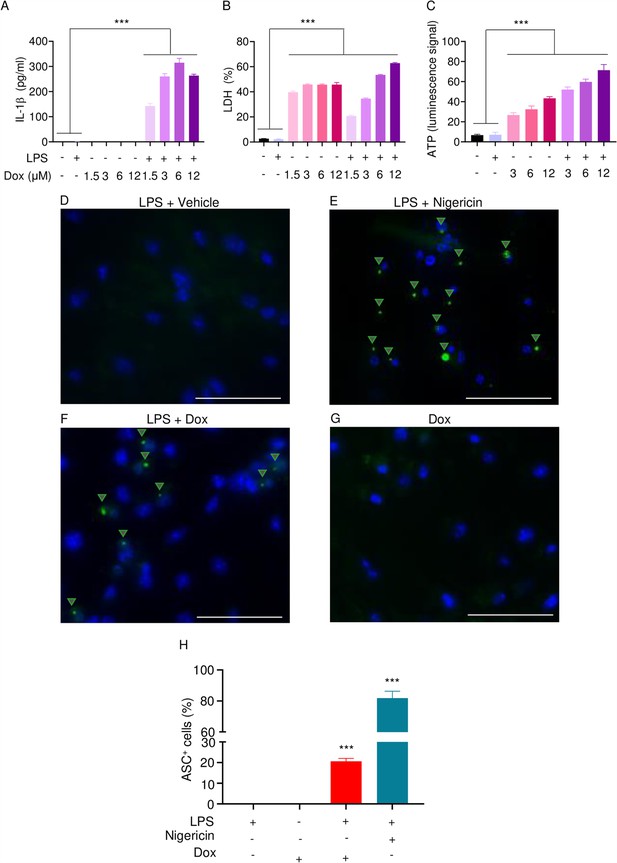
Doxorubicin activates inflammasomes and causes macrophage pyroptosis.
WT bone marrow-derived macrophages (BMMs) were left untreated or primed with LPS for 3 hr, then treated with various doxorubicin concentrations for 16 hr. IL-1β (A), LDH (B), and ATP (C) in the conditioned media were measured by enzyme linked immunosorbent assay (ELISA), the cytotoxicity detection kit, or ATP detection kit, respectively. WT BMMs from ASC-citrine mice were primed with 100 ng/ml LPS for 3 hr and treated or not with 15 µM nigericin for 30 min or 10 µM doxorubicin for 16 hr (D–F). Non-primed cells were also treated with 10 µM doxorubicin for 16 hr (G). Scale bars: 50 µm. ASC specks were visualized under fluorescence microscopy and quantified using ImageJ. Quantitative data (H). Data are mean ± SEM from experimental triplicates and represent at least two independent experiments. ***p<0.001 vs. untreated- or LPS-treated cultures. One-way ANOVA. ASC, apoptosis-associated speck-like protein containing a CARD; ATP, adenosine triphosphate; Dox, doxorubicin; IL-1β, interleukin-1β; LDH, lactate dehydrogenase; LPS, lipopolysaccharide; WT, wild-type.
-
Figure 3—source data 1
IL-1β, lactate dehydrogenase (LDH), and ATP analysis of bone marrow-derived macrophages (BMMs) supernatant in Figure 3A–C.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig3-data1-v1.xlsx
-
Figure 3—source data 2
ASC+ cells analysis in Figure 3H.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig3-data2-v1.xlsx
To reinforce the proposition that doxorubicin activates inflammasomes, we assessed the effects of this drug on the formation of ASC specks, a readout of inflammasome-activated states (SAtutza et al., 2013). As anticipated, LPS induced ASC speck formation only in the presence of nigericin, a well-known trigger of NLRP3 inflammasome assembly signals (Figure 3D, E, and H). Likewise, doxorubicin induced ASC speck formation only in LPS-primed BMMs (Figure 3F, G, and H). Next, we performed immunoblotting to analyze the expression of NLRP3 and AIM2 since these sensors assemble inflammasomes in response to DAMPs such as ATP and DNA, which were released by doxorubicin-damaged cells. We also determined the expression of other key components of these pathways such as caspase-1 and gasdermins. LPS induced the expression of NLRP3, but not AIM2, caspase-1, caspase-3, GSDMD, and GSDME (Figure 4). Levels of caspase-1 (p10) and GSDMD (p30) fragments, which are generated upon inflammasome activation, were higher in cells treated with LPS+doxorubicin compared to doxorubicin alone (Figure 4, Figure 4—figure supplement 2). GSDMD (p10) and GSDME (p35) fragments, which are proteolytically generated by caspase-3 (Figure 4—figure supplement 1A and B), were also detected, but their abundance was comparable between cells exposed to LPS+doxorubicin and doxorubicin alone (Figure 4 and Figure 4—figure supplement 2). Together, these results suggest that doxorubicin activates both caspase-1 and caspase-3, which cleave GSDMD and GSDME, ultimately, causing pyroptosis and IL-1β release.
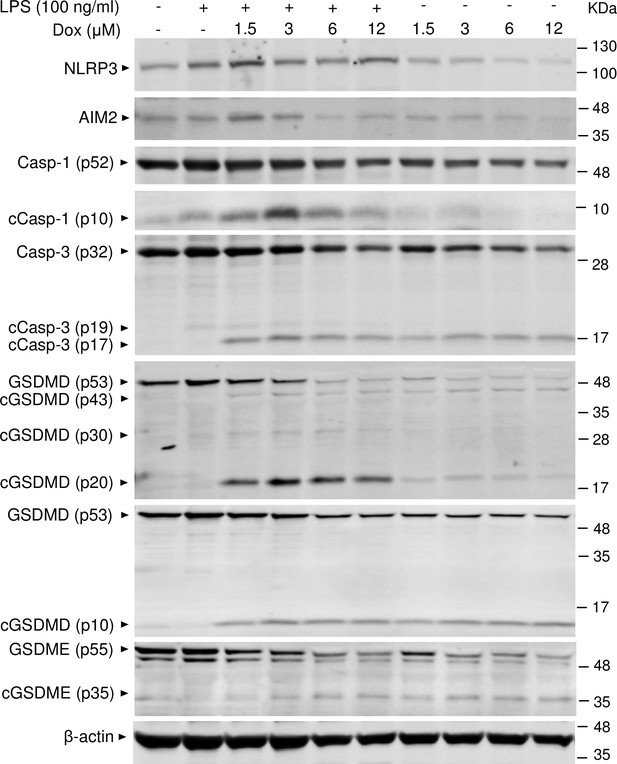
Doxorubicin activates inflammasome-dependent and -independent pathways in macrophages.
WT bone marrow-derived macrophages (BMMs) were left untreated or primed with LPS for 3 hr, then treated with various doxorubicin concentrations for 16 hr. Whole cell lysates were analyzed by immunoblotting. Data are representative of at least three independent experiments. AIM2, absent in melanoma 2; cCasp, cleaved caspase; cGSDM, cleaved gasdermin; LPS, lipopolysaccharide; Dox, doxorubicin; WT, wild-type.
-
Figure 4—source data 1
Original file for the western blot analysis in Figure 4 (NLRP3).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig4-data1-v1.zip
-
Figure 4—source data 2
Original file for the western blot analysis in Figure 4 (AIM2).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig4-data2-v1.zip
-
Figure 4—source data 3
Original file for the western blot analysis in Figure 4 (caspase-1).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig4-data3-v1.zip
-
Figure 4—source data 4
Original file for the western blot analysis in Figure 4 (cleaved caspase-1).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig4-data4-v1.zip
-
Figure 4—source data 5
Original file for the western blot analysis in Figure 4 (caspase-3/cleaved caspase-3).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig4-data5-v1.zip
-
Figure 4—source data 6
Original file for the western blot analysis in Figure 4 (gasdermin D [GSDMD]/cleaved GSDMD).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig4-data6-v1.zip
-
Figure 4—source data 7
Original file for the western blot analysis in Figure 4 (gasdermin D [GSDMD]/cleaved GSDMD [(p10])).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig4-data7-v1.zip
-
Figure 4—source data 8
Original file for the western blot analysis in Figure 4 (GSDME/cleaved GSDME).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig4-data8-v1.zip
-
Figure 4—source data 9
Original file for the western blot analysis in Figure 4 (β-actin).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig4-data9-v1.zip
-
Figure 4—source data 10
Original images of the relevant western blot analysis (NLRP3, AIM2, caspase-1/cleaved caspase-1, caspase-3/cleaved caspase-3, gasdermin D [GSDMD]/cleaved GSDMD, GSDME/cleaved GSDME, and β-actin) with highlighted bands and sample labels in Figure 4.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig4-data10-v1.zip
Doxorubicin activates inflammasome-dependent and -independent pathways, and causes NETosis in vitro
To further support the conclusion that doxorubicin induced the formation of NETs, first, we analyzed the expression of some key players directly or indirectly involved in this process. Incubation of mouse bone marrow neutrophils with LPS resulted in increased NLRP3 expression (Figure 5A). The abundance of caspase-1 (p10) and GSDMD (p30) was indistinguishable between cells exposed to LPS+doxorubicin and doxorubicin alone, likely as the result of cell death as the levels of β-actin used as loading control were markedly reduced in samples from these cells (Figure 5A and Figure 5—figure supplement 1). Levels of GSDMD (p10) and GSDME (p35) fragments were also similar between LPS+doxorubicin compared to doxorubicin alone (Figure 5A, Figure 4—figure supplement 1, Figure 5—figure supplement 1). LPS also induced IL-1β secretion, a response that was enhanced by doxorubicin in a dose-dependent manner (Figure 5B). LPS reduced baseline as well as doxorubicin-induced LDH release (Figure 5C). In sum, unchallenged neutrophils released LDH, a response that aligned with the presence of functional caspase-3, GSDMD, and GSDME fragments in these cells. However, LPS was required for optimal NLRP3 expression, caspase-1 activation, and IL-1β production by neutrophils. Although neutrophils secreted higher levels of IL-1β in response to doxorubicin, they were highly sensitive to the cytotoxic effects of this drug.
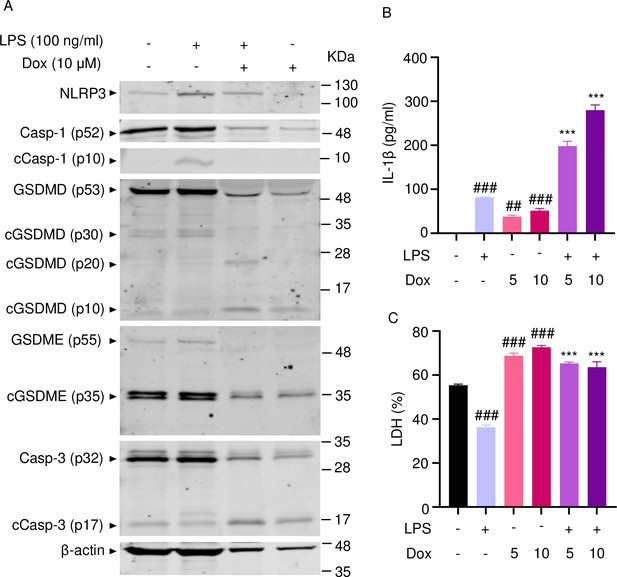
Doxorubicin activates inflammasome-dependent and -independent pathways in neutrophils.
WT mouse bone marrow neutrophils were left untreated or primed with LPS for 3 hr, then treated with various doxorubicin concentrations for 16 hr. Whole cell lysates were analyzed by immunoblotting. Blots are representative of at least three independent experiments (A), IL-1β (B), and LDH (C) in the conditioned media were measured by enzyme linked immunosorbent assay (ELISA) and the cytotoxicity detection kit, respectively. Data are mean ± SEM from experimental triplicates and are representative of at least two independent experiments. ***p<0.001 vs. LPS; ##p<0.01, ###p<0.001 vs. untreated cultures. One-way ANOVA was used. cCasp, cleaved caspase; cGSDM, cleaved gasdermin; IL-1β, interleukin-1β; LDH, lactate dehydrogenase; LPS, lipopolysaccharide; Dox, doxorubicin.
-
Figure 5—source data 1
Original file for the western blot analysis in Figure 5A (NLRP3).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig5-data1-v1.zip
-
Figure 5—source data 2
Original file for the western blot analysis in Figure 5A (caspase-1/cleaved caspase-1).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig5-data2-v1.zip
-
Figure 5—source data 3
Original file for the western blot analysis in Figure 5A (gasdermin D [GSDMD]/cleaved GSDMD).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig5-data3-v1.zip
-
Figure 5—source data 4
Original file for the western blot analysis in Figure 5A (GSDME/cleaved GSDME).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig5-data4-v1.zip
-
Figure 5—source data 5
Original file for the western blot analysis in Figure 5A (caspase-3/cleaved caspase-3).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig5-data5-v1.zip
-
Figure 5—source data 6
Original file for the western blot analysis in Figure 5A (β-actin).
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig5-data6-v1.zip
-
Figure 5—source data 7
Original images for the western blot analysis (NLRP3, caspase-1/cleaved caspase-1, gasdermin D [GSDMD]/cleaved GSDMD, GSDME/cleaved GSDME, caspase-3/cleaved caspase-3, and β-actin) with highlighted bands and sample labels in Figure 5A.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig5-data7-v1.zip
-
Figure 5—source data 8
IL-1β and lactate dehydrogenase (LDH) analysis of bone marrow neutrophils supernatant in Figure 5B and C.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig5-data8-v1.xlsx
Next, we performed immunofluorescence to visualize NET components in neutrophils treated with vehicle or this drug for 16 hr. Consistent with in vivo results, Cit-H3 and MPO were detected in cells treated with doxorubicin but not with vehicle (Figure 6A). Accordingly, levels of cfDNA were higher in doxorubicin-exposed cultures compared to untreated or cultures treated with LPS (Figure 6B). To determine the biological relevance of cfDNA while modeling the in vivo bone microenvironment, we assessed the impact of degrading cfDNA with DNase I on IL-1β release by cultured whole bone marrow cells, which comprised various cell types, including neutrophils and macrophages. IL-1β levels were higher in cells treated with LPS and doxorubicin compared to LPS, an outcome that was inhibited by DNase I (Figure 6C). Thus, doxorubicin promoted IL-1β release, a response that correlated with its effects on NET formation and abundance of cfDNA.
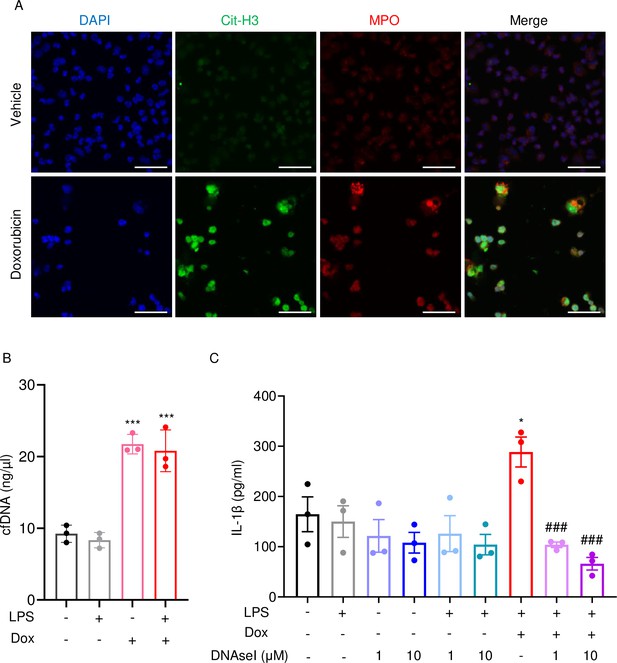
Doxorubicin causes NETosis in vitro.
Wild-type (WT) mouse bone marrow neutrophils were left untreated or treated with 10 µM doxorubicin for 16 hr (A). Cit-H3 and MPO were analyzed by immunofluorescence. Scale bars: 50 μm. Images are representative of at least three independent experiments. Neutrophils were left untreated or primed with LPS for 3 hr, then treated with 10 µM doxorubicin for 16 hr. cfDNA in the conditioned medium was extracted and quantified (B). Neutrophils were left untreated or primed with LPS for 3 hr, then treated with 10 µM doxorubicin and/or DNase I for 16 hr. IL-1β in the conditioned media was measured by enzyme linked immunosorbent assay (ELISA) (C). Data are mean ± SEM from experimental triplicates and are representative of at least two independent experiments. *p<0.05; **p<0.01; ***p<0.001 vs. LPS; #p<0.05, ###p<0.001 vs. LPS+Dox. One-way ANOVA was used. Dox, doxorubicin; cfDNA, cell-free DNA; Cit-H3, citrullinated histone H3; MPO, myeloperoxidase.
-
Figure 6—source data 1
Cell-free DNA (cfDNA) and IL-1β analysis of bone marrow neutrophils supernatant in Figure 6B–C.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig6-data1-v1.xlsx
AIM2 and NLRP3 inflammasomes are involved in bone-damaging effects of doxorubicin
We hypothesized that doxorubicin induced IL-1β and IL-18 release by activating the AIM2 and NLRP3 inflammasomes as blood levels of their activators (DNA, ATP) were increased in response to doxorubicin administration (Figure 2F, I, J). To test this idea, we measured the effects of this drug on IL-1β and LDH release by WT, Aim2-/-, Nlrp3-/-, Aim2-/-; Nlrp3-/-, or Casp1-/- BMMs and neutrophils. LPS induction of IL-1β and LDH release by BMMs required doxorubicin, a response that was significantly reduced in Aim2-/- or Nlrp3-/- cells (Figure 7A and B). LPS induced IL-1β secretion by neutrophils, an outcome that was enhanced by doxorubicin, and comparably attenuated in all mutants (Figure 7C and D). Differences in LDH release were marginal, perhaps because baseline levels of this readout were high, consistent with the short lifespan of these cells in vitro. Because inflammation leads to bone loss, we assessed bone outcomes of 10-week-old WT, Aim2-/- and/or Nlpr3-/-, 4 weeks after receiving a single dose of 5 mg/kg doxorubicin or vehicle. Administration of doxorubicin to WT mice caused bone loss associated with increased OC number and surface (Figure 7E–I and Figure 7—figure supplement 1A–C), consistent with the results shown above (Figure 1). These responses were reduced slightly in Nlrp3-deficient mice, but significantly in Aim2 null mice. Aim2-/- male mice lost bone comparably to Aim2-/-;Nlrp3-/- and casp1-/- counterparts (Figure 7E–G). Similar trends in bone changes were observed in female mice, though Aim2-/- mice were more osteopenic than casp1-/- mice (Figure 7—figure supplement 2A–D). Collectively, our results suggest that the AIM2 and NLRP3 inflammasomes participate to various extent in doxorubicin bone-damaging effects. They also suggest that inflammasome-independent actions of this drug on bone are not negligible.
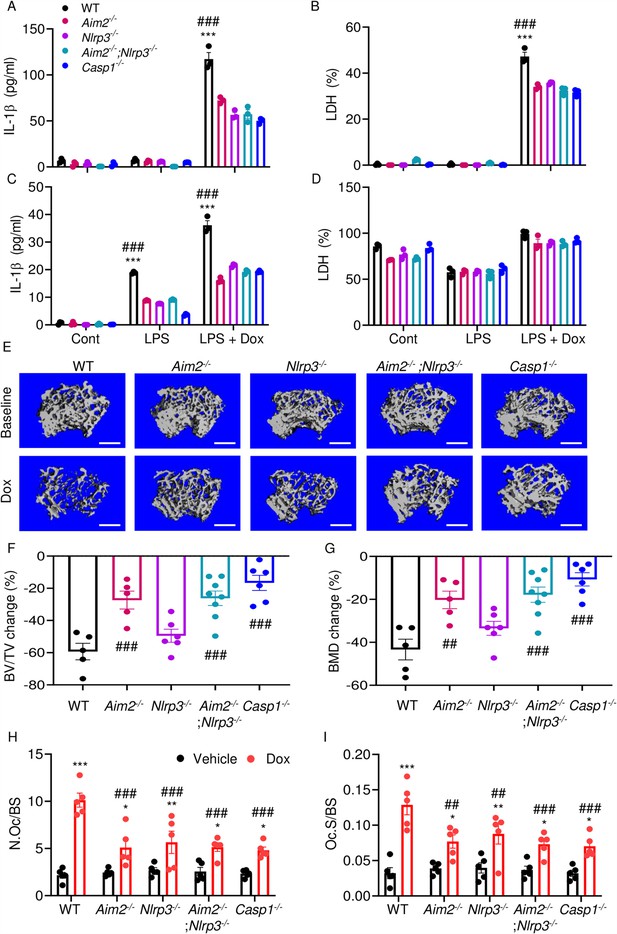
AIM2 and NLRP3 inflammasomes are involved in bone-damaging effects of doxorubicin.
WT, Aim2-/-, Nlrp3-/-, Aim2-/-;Nlrp3-/- or Casp1-/- bone marrow-derived macrophages (BMMs) (A, B) and neutrophils (C, D) were left untreated or primed with LPS for 3 hr, then exposed or not to 10 µM doxorubicin for 16 hr. IL-1β (A, C) and LDH (B, D) in the conditioned media were measured by enzyme linked immunosorbent assay (ELISA) and the cytotoxicity detection kit, respectively. Femurs from male mice were analyzed by VivaCT before (baseline) and 4 weeks after a single intraperitoneal injection of 5 mg/kg doxorubicin (E–G). Cross sections of 3D reconstructions. Scale bars: 500 μm (E). BV/TV changes (F). BMD changes (G). Femurs harvested from different genotypes of male mice were analyzed 4 weeks after a single intraperitoneal injection of vehicle or doxorubicin (H, I). Specimens were stained for tartrate-resistant acidic phosphatase (TRAP) activity. N.Oc/BS (H). Oc.S/BS (I). Data are mean ± SEM from experimental triplicates and are representative of at least two independent experiments (A–D); n=5–8 mice/group (E–I). Data are mean ± SEM. *p<0.05; **p<0.01; ***p<0.001 vs. control, LPS, or vehicle; ##p<0.01, ###p<0.001 vs. other genotypes or WT treated with Dox. Two-way ANOVA (A–D, H–I) and one-way ANOVA (F–G) were used. AIM2, absent in melanoma 2; BMD bone mineral density; BV/TV, bone volume/total volume; casp1, caspase-1; Cont, control; Dox, doxorubicin; IL-1β, interleukin-1β; LDH, lactate dehydrogenase; LPS, lipopolysaccharide; N.Oc/BS, OC number/bone surface; Oc.S/BS; OC, osteoclast; WT, wild-type.
-
Figure 7—source data 1
IL-1β and lactate dehydrogenase (LDH) analysis of different genotypes of bone marrow-derived macrophages (BMMs) and bone marrow neutrophils supernatant in Figure 7A–D.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig7-data1-v1.xlsx
-
Figure 7—source data 2
Micro-computed tomography (µCT) analysis of different genotypes of mice in Figure 7F and G.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig7-data2-v1.xlsx
-
Figure 7—source data 3
Tartrate-resistant acidic phosphatase (TRAP) staining analysis of different genotypes of mice in Figure 7H and I.
- https://cdn.elifesciences.org/articles/92885/elife-92885-fig7-data3-v1.xlsx
Discussion
We found that the AIM2 inflammasome and the NLRP3 inflammasome to a lesser extent played an important role in bone-damaging effects of doxorubicin. The comparable bone phenotype of Aim2-/-;Nlrp3-/- and casp1-/- mice suggested that the AIM2 and NLRP3 inflammasomes were the primary mediators of doxorubicin actions. Because doxorubicin activates several pathways, some of which interact or overlap with inflammasome functions (e.g. senescence factors), the remaining bone loss in compound mutant mice was expected. The interplay among these pathways may have accounted for the sex differences in the bone outcomes of inflammasome insufficiency as residual doxorubicin-induced bone loss was higher in Aim2-/- and Aim2-/-;Nlrp3-/- female mice than in male counterparts. Sexual dimorphic actions of inflammasomes were not unprecedented as uneven severity of atherosclerosis was found in male and female Nlrp3-deficient mice with gonadal insufficiency (Chen et al., 2020) and sex-dependent differential activation of AIM2 and NLRP3 inflammasomes in macrophages from systemic lupus erythematous had been reported (Yang et al., 2015). Although the impacts of doxorubicin on bone pathology are complex, including its direct actions on bone cells (Yao et al., 2020; Chai et al., 2014; Rana et al., 2013; Zhou and Kuai, 2020), our investigation focused on immune cells, and found that the inactivation of inflammasomes is sufficient to attenuate the drug’s bone-damaging effects.
Doxorubicin causes neutropenia, lymphopenia, and anemia in patients (Gatti et al., 2018; Boccia et al., 2022). Consistent with the clinical situation, administration of doxorubicin to mice caused leukopenia, which correlated with lymphocytopenia and monocytopenia, and was associated with fluctuations in neutrophil counts. Since neutrophils were highly sensitive to the cytotoxic effects of doxorubicin, the transient neutrophilic effects of this drug in mice may be the result of emergency granulopoiesis, a physiological response that is rapidly triggered to restore adequate neutrophil number. This leukopenic outcome was consistent with increased serum levels of cell death-associated DAMPS (ATP and cfDNA) and our results showing that doxorubicin activated the effectors of apoptosis, pyroptosis, and NETosis, including caspase-1, caspase-3, GSDMD, and GSDME. Since pyroptosis and NETosis promote inflammation and immune responses, we argued that they accounted for the cytokinemic effects of doxorubicin. Other studies, however, found that doxorubicin reduced NET formation in cancer models and by human neutrophils in vitro (Lu et al., 2021; Khan et al., 2019). This discrepancy may be due to differences in the experimental models and cell context-dependent actions of doxorubicin. Other limitations of our study include its focus on: (i) macrophages and neutrophils while oversighting lymphocytes or even other cells bone microenvironment such as mesenchymal and adipocytes whose fate is affected by this drug (Fan et al., 2018; Fan et al., 2017; Wang et al., 2012; Buttiglieri et al., 2011), which were also targeted by doxorubicin; (ii) immune cells without assessing the direct effects of doxorubicin on bone cells, as noted above; and (iii) the use of the tumor-free model as immune responses can differ significantly in the absence or presence of cancer cells. Despite these limitations, our findings point to a novel mechanism of action for doxorubicin in bone.
DNA accumulates in the cytoplasm as the result of genomic instability, damaged mitochondria, or lysed intracellular pathogens. Extracellular DNA from pathogens, NETotic, or pyroptotic cells can be internalized and culminate in the cytoplasm. In either case, sensors such as AIM2 detect mislocated DNA in the cytoplasm and trigger inflammatory responses (Boccia et al., 2022; Blayney and Schwartzberg, 2022; Nakazawa et al., 2016; Jeong et al., 2021). This view was consistent with our data showing a correlation between the levels of extracellular DNA and IL-1β as well as by the inhibition of IL-1β secretion by DNase I. We also found that doxorubicin activated the NLRP3 inflammasome and induced the release of ATP, a well-known activator of the NLRP3 inflammasome. Whether doxorubicin activated the NLRP3 inflammasome directly by perturbing the plasma membrane or indirectly via generation of secondary signals such as ATP remained unclear.
By showing that inflammasomes are key players in bone loss caused by doxorubicin, this work advances our knowledge on potential mechanisms of action of this drug on this tissue (Figure 8). This insight is difficult to get in clinical situations because this chemotherapeutic is employed not alone but in conjunction with other medications (Zimny, 1988; Svendsen et al., 2017; Müller et al., 2020).
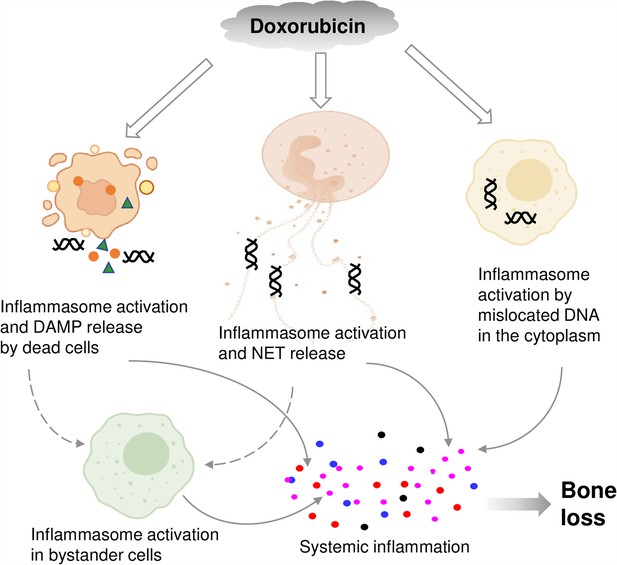
Graphical abstract.
Double line arrows: direct effects of doxorubicin on target cells. Solid line arrows: direct contribution to systemic inflammation. Broken line arrows: indirect contribution to systemic inflammation.
© 2024, BioRender Inc. Figure 8 was created using BioRender, and is published under a CC BY-NC-ND license. Further reproductions must adhere to the terms of this license.
Materials and methods
Animals
WT, R26-CAG-ASC-citrine (030744), Aim2-/- (013144), and Nlrp3-/- (021302) mice were purchased from The Jackson Laboratory (Sacramento, CA, USA). Casp1-/- were kindly provided by Dr. Thirumala-Devi Kanneganti (St. Jude Children’s Research Hospital). Aim2-/- mice and Nlrp3-/- mice were intercrossed to generate Aim2-/-;Nlrp3-/- mice. All mice were on the C57BL/6J background and mouse genotyping was performed by PCR. All procedures were approved by the Institutional Animal Care and Use Committee (IACUC) of Washington University School of Medicine in St. Louis. All experiments were performed in accordance with the relevant guidelines and regulations described in the IACUC-approved protocol 22-0335.
Doxorubicin administration and VivaCT analysis
Request a detailed protocolThe femurs of 10-week-old mice were analyzed by VivaCT 2 weeks before (baseline) and 4 weeks after a single intraperitoneal injection (i.p.) of 5 mg/kg doxorubicin (Sigma-Aldrich, MO, USA) formulated in H2O at 1 mg/ml or vehicle. For bone analysis, mice were anesthetized with isofluorane and trabecular volume in the distal femoral metaphysis of the right leg was measured using VivaCT 40 (Scanco Medical AG, Zurich, Switzerland) set at 70 kVp, 114 μA, and 20 μm resolution as previously described (Yao et al., 2020). For the trabecular bone compartment, contours were traced on the inside of the cortical shell using 2D images of the femoral metaphysis. The end of the growth plate region was used as a landmark to establish a consistent location for starting analysis, and the next 50 slices were analyzed. The following trabecular parameters are reported for all VivaCT experiments: bone volume over total volume, trabecular number, trabecular thickness, trabecular separation, and volumetric bone mineral density.
Histomorphometry
Request a detailed protocolFor static histomorphometry, the femurs were fixed in 10% neutral buffered formalin overnight and decalcified in 14% (wt/vol) EDTA, pH 7.2, for 10–14 days at room temperature. Fixed femurs were embedded in paraffin, sectioned at 5 μm thicknesses, and mounted on glass slides. The sections were stained with tartrate-resistant acidic phosphatase as described previously (Wang et al., 2018). For dynamic histomorphometry, mice were i.p. injected with 10 mg/kg calcein green (Sigma-Aldrich, MO, USA) and 6 days later with 50 mg/kg alizarin red (Sigma-Aldrich, MO, USA). Mice were euthanized 2 days after the second injection. The tibias were collected and fixed in 10% neutral buffered formalin overnight, embedded in methyl methacrylate, and sectioned at 7–10 μm. Images were taken using a Nanozoomer 2.0 HT whole slide scanner (Hamamatsu Photonics, Shizuoka, Japan) at ×20 magnification. Bioquant Osteo software (v18.2.6; Bioquant Image Analysis Corp, TN, USA) was used for image analysis. Measurements of dynamic bone histomorphometry were calculated from fluorochrome double labels at the endocortical surfaces as previously described (Xiao et al., 2020).
Serum assays
Request a detailed protocolBlood was collected by cardiac puncture and was allowed to clot at room temperature. Serum obtained after centrifugation at 2000×g for 10 min was used for various assays. Cytokine and chemokine levels were measured by V-PLEX Plus Proinflammatory Panel 1 Mouse Kit (Meso Scale Diagnostics, MD USA), except IL-18, which was analyzed by enzyme linked immunosorbent assay (ELISA) kit (Sigma-Aldrich, MO, USA). The levels of Cit-H3 and MPO were determined by ELISA kits (Abcam, MA, USA, and Cayman, MI, USA).
Peripheral blood analysis
Request a detailed protocolMouse blood was collected by cardiac puncture in the EDTA-containing tubes. Complete blood counts were performed by the Washington University School of Medicine as previously described (Wang et al., 2017).
Cell cultures
Request a detailed protocolMurine primary BMMs were obtained by culturing mouse bone marrow cells in culture media containing a 1:10 dilution of supernatant from the fibroblastic cell line CMG 14-12 as a source of macrophage colony-stimulating factor, a mitogenic factor for BMMs, for 4–5 days in a 15 cm dish as previously described (Takeshita et al., 2000; Wang et al., 2020). Briefly, nonadherent cells were removed by vigorous washes with PBS, and adherent BMMs were detached with trypsin-EDTA and cultured in culture media containing a 1:10 dilution of CMG for various experiments. Murine primary neutrophils were isolated by collecting bone marrow cells and subsequently over a discontinuous Percoll (Sigma-Aldrich, MO, USA) gradient as described previously (Sun et al., 2022). Briefly, all bone marrow cells from femurs and tibias were washed by PBS and then resuspended in 2 ml PBS. Cell suspension was gently layered on top of gradient (72% Percoll, 64% Percoll, 52% Percoll) and centrifuged at 1545×g for 30 min at room temperature. After carefully discarding the top two cell layers, the third layer containing neutrophils was transferred to a clean 15 ml tube. Cells were washed and counted, then plated at a density of 1×105 cells/well in 96-well plate or 5×106 cells/well in six-well plate for 1 hr followed by various experiments. For all in vitro experiments except otherwise specified, BMMs were plated at 2×104 cells per well on a 96-well plate or 106 cells per well on a six-well plate overnight. Neutrophils were plated at 105 cells per well on a 96-well plate or 5×106 cells per well on a six-well plate for 1 hr prior to treatment. BMMs and neutrophils were primed with 100 ng/ml LPS (Sigma-Aldrich, MO, USA) for 3 hr, then with different concentrations of doxorubicin (Sigma-Aldrich, MO, USA) as indicated for 16 hr. Conditioned media were collected for the analysis of IL-1β and LDH. Cell lysates were collected for protein expression analysis by western blot as described below.
Western blot analysis
Request a detailed protocolCell extracts were prepared by lysing cells with RIPA buffer (50 mM Tris, 150 mM NaCl, 1 mM EDTA, 0.5% NaDOAc, 0.1% SDS, and 1.0% NP-40) plus phosphatase and protease inhibitors (GenDEPOT, TX, USA). Protein concentrations were determined by the Bio-Rad Laboratories method (Bio-Rad, CA, USA), and equal amounts of proteins were subjected to SDS-PAGE gels (12% or 15%) as previously described (Wang et al., 2021). Proteins were transferred onto nitrocellulose membranes and incubated with antibodies against GSDMD (1;1000; Abcam, MA, USA; ab219800; ab209845), GSDME (1;1000; Abcam, MA, USA; ab215191), caspase-1 (1;1 000; Abcam, MA, USA; ab179515), caspase-3 (1:1000; Cell Signaling Technologies, MA, USA; 9662S), NLRP3 (1:1000; AdipoGen, CA, USA; AG20B0014C), AIM2 (1:1000; Cell Signaling Technologies, MA, USA; 63660S) or β-actin (1:2000; Santa Cruz Biotechnology, TX, USA; SC47778) overnight at 4°C followed by incubation for 1 hr with secondary goat anti-mouse IRDye 800 (Li-COR Biosciences, NE, USA; 926-32210) or goat anti-rabbit Alexa Fluor 680 (Li-COR Biosciences, NE, USA; 926-68071) respectively. The results were visualized using the Odyssey infrared imaging system (LI-COR Biosciences, NE, USA).
LDH assay and IL-1β ELISA
Request a detailed protocolCell death was assessed by the release of LDH in conditioned medium using LDH cytotoxicity detection kit (TaKaRa, CA, USA). IL-1β levels in conditioned media were measured by an ELISA kit (eBiosciences, NY, USA).
ASC specks assay
Request a detailed protocolASC-citrine-WT BMMs were plated at 104 cells per well on a 16-well glass plate overnight. Cells were primed with LPS for 3 hr followed by 15 μM nigericin (AdipoGen, CA, USA) for 30 min or the indicated doxorubicin concentrations for 16 hr. Cells were washed with PBS, fixed with 4% paraformaldehyde buffer for 10 min at room temperature, then counterstained with Fluoro-gel II containing DAPI (Fluoro-Gel, Fisher Scientific Intl INC, PA, USA). ASC-citrine photographs were taken using ZEISS microscopy (Carl ZEISS Industrial Metrology, MN, USA). Quantification of ASC specks was carried out using ImageJ.
Immunofluorescence
Request a detailed protocolIsolated neutrophils were plated at 105 cells per well on a 16-well glass plate for 1 hr. Cells were primed with LPS for 3 hr, treated with doxorubicin for 16 hr, washed with PBS, and fixed with 4% paraformaldehyde buffer for 10 min at room temperature. Cells were permeabilized with 0.2% Triton in PBS for 20 min, blocked with 0.2% Triton and 1% BSA in PBS for 30 min, and were incubated with Cit-H3 antibody (1:1000; Abcam, MA, USA; ab5103) and MPO (1;1000; Abcam, MA, USA; ab90810) overnight at 4°C in blocking buffer, followed by incubation with secondary antibody (Alexa Fluor 594, 1:2000; Life Technologies, CA, USA; A11020; A27034) for 30 min. Cells were counterstained with Fluoro-gel II containing DAPI (Fluoro-Gel, Fisher Scientific Intl INC, PA, USA). Immunostaining images were taken using a Leica inverted microscope with a TCS SPEII confocal module and processed using LAS X software (Leica Microsystems Inc, IL, USA).
cfDNA assay
Request a detailed protocolcfDNA in the conditioned cell culture medium was extracted using NucleoSpin Gel and PCR Clean-up kit (Takara, Duren, Germany), and quantified with Nanodrop (Thermo Fisher Scientific, MA, USA). cfDNA in the serum was purified and measured using Qubit by Washington University School of Medicine Hope Center DNA/RNA purification Core or using SYBR Green (Thermo Fisher Scientific, MA, USA) as previously described (Goldshtein et al., 2009; Villalba-Campos et al., 2016).
ATP assay
Request a detailed protocolATP levels in conditioned media and serum were measured by RealTime-Glo Extracellular ATP Assay kit (Promega, Madison, WI, USA).
Statistical analysis
Request a detailed protocolStatistical analysis was performed using the Student’s t-test, one-way ANOVA with Tukey’s multiple comparisons test, or two-way ANOVA with Tukey’s multiple comparisons test, Dunnett’s multiple comparisons test, or Sidak’s multiple comparisons test using the GraphPad Prism 9.0 software. Values are expressed as mean ± SEM. *p<0.05 was considered statistically significant.
Data availability
All data generated or analysed during this study are included in the manuscript and supporting files; source data files have been provided for all figures.
References
-
De novo CIAS1 mutations, cytokine activation, and evidence for genetic heterogeneity in patients with neonatal-onset multisystem inflammatory disease (NOMID): a new member of the expanding family of pyrin-associated autoinflammatory diseasesArthritis and Rheumatism 46:3340–3348.https://doi.org/10.1002/art.10688
-
The cytosolic DNA sensor cGAS recognizes neutrophil extracellular trapsScience Signaling 14:eaax7942.https://doi.org/10.1126/scisignal.aax7942
-
Chemotherapy-induced neutropenia and emerging agents for prevention and treatment: a reviewCancer Treatment Reviews 109:102427.https://doi.org/10.1016/j.ctrv.2022.102427
-
Doxorubicin decreases the repair of radiation-induced DNA damageInternational Journal of Radiation Biology 57:55–64.https://doi.org/10.1080/09553009014550341
-
Cytotoxic chemotherapy increases sleep and sleep fragmentation in non-tumor-bearing miceBrain, Behavior, and Immunity 47:218–227.https://doi.org/10.1016/j.bbi.2014.11.001
-
The aging effect of chemotherapy on cultured human mesenchymal stem cellsExperimental Hematology 39:1171–1181.https://doi.org/10.1016/j.exphem.2011.08.009
-
Successful skeletal aging: a marker of low fracture risk and longevityThe Study of Osteoporotic Fractures (SOF). J Bone Miner Res 24:134–143.https://doi.org/10.1359/jbmr.080813
-
Loss of hip BMD in older men: the osteoporotic fractures in men (MrOS) studyJournal of Bone and Mineral Research 24:1728–1735.https://doi.org/10.1359/jbmr.090419
-
Change in hip bone mineral density and risk of subsequent fractures in older menJournal of Bone and Mineral Research 27:2179–2188.https://doi.org/10.1002/jbmr.1671
-
Molecular stress-inducing compounds increase osteoclast formation in a heat shock factor 1 protein-dependent mannerThe Journal of Biological Chemistry 289:13602–13614.https://doi.org/10.1074/jbc.M113.530626
-
Sex-specific effects of the nlrp3 inflammasome on atherogenesis in ldl receptor-deficient miceJACC. Basic to Translational Science 5:582–598.https://doi.org/10.1016/j.jacbts.2020.03.016
-
Management of cancer treatment-induced bone lossNature Reviews. Rheumatology 9:365–374.https://doi.org/10.1038/nrrheum.2013.36
-
Combination breast cancer chemotherapy with doxorubicin and cyclophosphamide damages bone and bone marrow in a female rat modelBreast Cancer Research and Treatment 165:41–51.https://doi.org/10.1007/s10549-017-4308-3
-
Genetic background influences susceptibility to chemotherapy-induced hematotoxicityThe Pharmacogenomics Journal 18:319–330.https://doi.org/10.1038/tpj.2017.23
-
New drugs for the treatment of cancer, 1990-2001The Israel Medical Association Journal 4:1124–1131.
-
A rapid direct fluorescent assay for cell-free DNA quantification in biological fluidsAnnals of Clinical Biochemistry 46:488–494.https://doi.org/10.1258/acb.2009.009002
-
Inflammasomes: mechanism of action, role in disease, and therapeuticsNature Medicine 21:677–687.https://doi.org/10.1038/nm.3893
-
Antitumor drug toxicity in tumor-free and tumor-bearing miceCancer Chemotherapy and Pharmacology 4:199–204.https://doi.org/10.1007/BF00254019
-
Recent developments in combination chemotherapy for colorectal and breast cancers with topoisomerase inhibitorsInternational Journal of Molecular Sciences 24:8457.https://doi.org/10.3390/ijms24098457
-
Neutrophil extracellular trap clearance by synovial macrophages in goutArthritis Research & Therapy 23:88.https://doi.org/10.1186/s13075-021-02472-4
-
Macrophage uptake of necrotic cell dna activates the aim2 inflammasome to regulate a proinflammatory phenotype in ckdJournal of the American Society of Nephrology 29:1165–1181.https://doi.org/10.1681/ASN.2017080863
-
Reduction of doxorubicin cytotoxicity by ouabain: correlation with topoisomerase-induced DNA strand breakage in human and hamster cellsCancer Research 48:725–730.
-
Simultaneous inhibition of breast cancer and its liver and lung metastasis by blocking inflammatory feed-forward loopsJournal of Controlled Release 338:662–679.https://doi.org/10.1016/j.jconrel.2021.08.047
-
Primary bone lymphoma: clinical presentation and therapeutic considerationsJournal of Bone Oncology 25:100326.https://doi.org/10.1016/j.jbo.2020.100326
-
The responses of macrophages in interaction with neutrophils that undergo netosisJournal of Autoimmunity 67:19–28.https://doi.org/10.1016/j.jaut.2015.08.018
-
Cross-sensitivity to topoisomerase II inhibitors in cytotoxic drug-hypersensitive Chinese hamster ovary cell linesCancer Research 47:1560–1565.
-
ASC speck formation as a readout for inflammasome activationMethods in Molecular Biology 1040:91–101.https://doi.org/10.1007/978-1-62703-523-1
-
NLRP3 inflammasome in cancer and metabolic diseasesNature Immunology 22:550–559.https://doi.org/10.1038/s41590-021-00886-5
-
Doxorubicin: an update on anticancer molecular action, toxicity and novel drug delivery systemsThe Journal of Pharmacy and Pharmacology 65:157–170.https://doi.org/10.1111/j.2042-7158.2012.01567.x
-
Identification and characterization of the new osteoclast progenitor with macrophage phenotypes being able to differentiate into mature osteoclastsJournal of Bone and Mineral Research 15:1477–1488.https://doi.org/10.1359/jbmr.2000.15.8.1477
-
Quantification of cell-free DNA for evaluating genotoxic damage from occupational exposure to car paintsJournal of Occupational Medicine and Toxicology 11:33.https://doi.org/10.1186/s12995-016-0123-8
-
Selective inhibition of the p38α MAPK-MK2 axis inhibits inflammatory cues including inflammasome priming signalsThe Journal of Experimental Medicine 215:1315–1325.https://doi.org/10.1084/jem.20172063
-
Parp1 hinders histone h2b occupancy at the nfatc1 promoter to restrain osteoclast differentiationJournal of Bone and Mineral Research 35:776–788.https://doi.org/10.1002/jbmr.3927
-
Doxorubicin area under the curve is an important predictor of neutropenia in dogs with naturally occurring cancersVeterinary and Comparative Oncology 17:147–154.https://doi.org/10.1111/vco.12455
-
Therapy-induced senescence drives bone lossCancer Research 80:1171–1182.https://doi.org/10.1158/0008-5472.CAN-19-2348
-
Doxorubicin restrains osteogenesis and promotes osteoclastogenesis in vitroAmerican Journal of Translational Research 12:5640–5654.
-
Mechanoreceptors in articular tissuesThe American Journal of Anatomy 182:16–32.https://doi.org/10.1002/aja.1001820103
Article and author information
Author details
Funding
National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR072623)
- Yousef Abu-Amer
National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR082192)
- Yousef Abu-Amer
Shriners Hospitals for Children (85109)
- Yousef Abu-Amer
National Institute of Arthritis and Musculoskeletal and Skin Diseases (P30 AR074992)
- Yousef Abu-Amer
National Institute of Arthritis and Musculoskeletal and Skin Diseases (R01-AR076758)
- Gabriel Mbalaviele
National Institute of Allergy and Infectious Diseases (R01-AI161022)
- Gabriel Mbalaviele
National Institute on Aging (R01 AG077732)
- Gabriel Mbalaviele
The funders had no role in study design, data collection and interpretation, or the decision to submit the work for publication.
Acknowledgements
We want to thank Dr. Deborah J Veis for reading this manuscript.
Version history
- Sent for peer review: October 6, 2023
- Preprint posted: October 9, 2023 (view preprint)
- Preprint posted: January 8, 2024 (view preprint)
- Preprint posted: March 8, 2024 (view preprint)
- Preprint posted: March 28, 2024 (view preprint)
- Version of Record published: April 11, 2024 (version 1)
Cite all versions
You can cite all versions using the DOI https://doi.org/10.7554/eLife.92885. This DOI represents all versions, and will always resolve to the latest one.
Copyright
© 2024, Wang et al.
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 532
- views
-
- 62
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.






