Mutualistic Relationships: Balancing metabolism and reproduction
Citrus huanglongbing – a disease known as ‘citrus cancer’ because of the devastating effect it can have on citrus plants – is caused by a bacterium called Candidatus Liberibacter asiaticus (CLas; Killiny, 2022). In Asia, this bacterium is primarily spread by a lice-like bug called Diaphorina citri. However, it is not possible to study CLas in the laboratory because efforts to culture it have been unsuccessful. Therefore, the most effective approach for preventing outbreaks of huanglongbing is to manage the population of D. citri (Wang et al., 2017).
Relationships between insects and plant pathogens – like that between D. citri and CLas – are common in the natural world, and many of these are mutually beneficial to both parties, though some are not (Eigenbrode et al., 2018). In a mutualistic or symbiotic relationship, the pathogen relies on the insect to help it spread from plant to plant, while infection by the pathogen can benefit the insect through an increase in fitness. To date, most research in this area has focused on relationships in which the pathogen is a virus (see, for example, Liu et al., 2014 on the spread of barley yellow dwarf virus by aphids, and Mao et al., 2019 on the spread of ice gall dwarf virus by leafhoppers), so less is known about mutualistic relationships involving bacteria.
Previous studies have revealed a mutualistic relationship in which CLas infection boosts the fitness of D. citri by increasing its ability to produce large numbers of offspring (fecundity), but the molecular dynamics driving this interaction were not fully understood (Pelz-Stelinski and Killiny, 2016; Ren et al., 2016). Now, in eLife, Yijing Cen, Xiaoge Nian and colleagues from South China Agricultural University, Shaoguan University, and Western Sydney University – including Jiayun Li as first author – report results that shed light on this interaction at the molecular level (Li et al., 2024).
Reproductive development in insects demands a significant energy supply (Yang et al., 2024), so exploring how CLas infection regulates and mobilizes energy metabolism in D. citri to improve fecundity is a promising avenue to explore. Insects store energy and fat as triglyceride and glycogen, and a process called AKH signaling (where AKH is short for adipokinetic hormone) has a crucial role in converting the former to diglyceride in order to release lipids, and the latter to trehalose to release energy (Arrese and Soulages, 2010).
First, Li et al. demonstrated that CLas infection significantly increases levels of triglyceride and glycogen, as well as lipid droplet size. These findings suggest that CLas-infected D. citri possess greater energy reserves and greater potential for energy mobilization, which may support the development of ovaries in females. Knocking down the gene for AKH (or its receptor) disrupted the mobilization of fat and this resulted in delayed ovary development, decreased egg production, and a reduction in the titer of CLas in the ovaries. These findings underscore the critical role of AKH and its receptor in managing the balance between energy metabolism and fecundity in D. citri.
To investigate how AKH signaling is regulated, Li et al. identified microRNAs that could potentially bind to AKH mRNA and prevent it being translated into protein. In vivo and in vitro experiments showed that a microRNA called miR-34 directly targets the 3’-untranslated region of the AKH receptor, therefore reducing the levels of this mRNA and the AKH receptor itself. Expression levels of miR-34 were lower in infected D. citri than in controls, suggesting that CLas may decrease levels of miR-34. Furthermore, treating infected D. citri with a synthetic microRNA that mimics the function of miR-34 led to outcomes similar to those observed when the gene for the AKH receptor was knocked down: delayed ovarian development, reduced egg production, and decreased levels of CLas in the ovaries (Figure 1). This finding represents the first instance of a host microRNA influencing AKH signaling to impact lipid metabolism and fecundity in infected D. citri. Additionally, the research points to an array of genes affected by the AKH signaling cascade, such as those associated with juvenile hormone signaling and two reproduction-related proteins called vitellogenin and vitellogenin receptor.
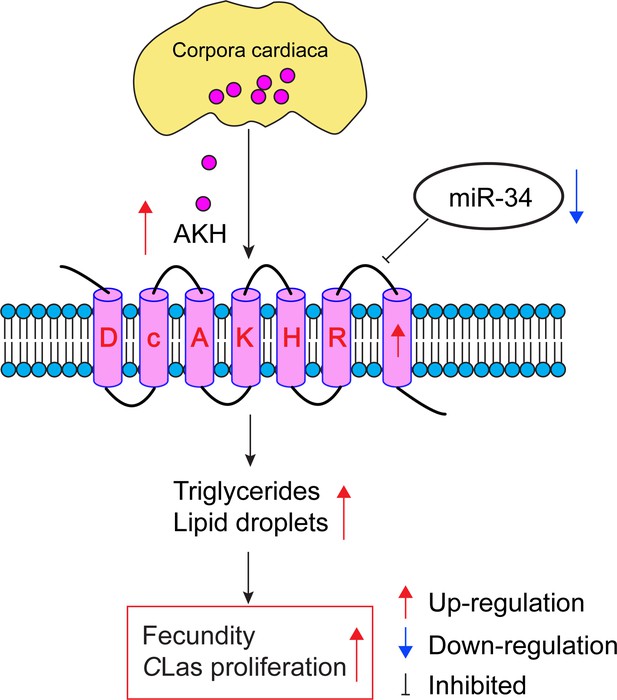
How infection by bacteria can modulate metabolism and increase reproduction in an insect.
When the lice-like insect D. citri is infected with CLas, the bacterium that causes huanglongbing in citrus plants, there is an increase in the production of adipokinetic hormone (AKH; pink circles; top) in the corpora cardiaca (yellow), which is part of the endocrine system of the insect. The AKH molecules bind to AKH receptors (pale pink barrel shapes), and the resulting increase in AKH receptor signaling leads to increased levels of triglyceride and increased numbers of lipid droplets (not shown). A microRNA called miR-34 usually inhibits AKH receptor signaling, but infection with CLas also results in the downregulation of miR-34. The increase in AKH receptor signaling caused by CLas infection increases the energy reserves available for reproduction, leading to increased fecundity for D. citri, which also aids the proliferation of CLas. CLas: Candidatus Liberibacter asiaticus.
The work of Li et al. in highlighting the role of AKH signaling in increasing the fecundity of D. citri that have been infected by CLas contributes to our understanding of the rapid spread of huanglongbing in the field. Future research should focus on elucidating the intricate interactions among endocrine signals such as hormones, neuropeptides and neurotransmitters, and their collective influence on the increased fecundity prompted by CLas infection. Undertaking these investigations will broaden our understanding of the complex relationships between plant pathogens and their insect vectors, and could potentially lead to new methods to control plant pathogen populations.
References
-
Insect fat body: energy, metabolism, and regulationAnnual Review of Entomology 55:207–225.https://doi.org/10.1146/annurev-ento-112408-085356
-
Insect-borne plant pathogens and their vectors: Ecology, evolution, and complex interactionsAnnual Review of Entomology 63:169–191.https://doi.org/10.1146/annurev-ento-020117-043119
-
Viral pathogens hitchhike with insect sperm for paternal transmissionNature Communications 10:955.https://doi.org/10.1038/s41467-019-08860-4
-
Better together: association with “Candidatus Liberibacter asiaticus” increases the reproductive fitness of its insect vector, Diaphorina citri (Hemiptera: Liviidae)Annals of the Entomological Society of America 109:371–376.https://doi.org/10.1093/aesa/saw007
-
Effects of Candidatus Liberibacter asiaticus on the fitness of the vector Diaphorina citriJournal of Applied Microbiology 121:1718–1726.https://doi.org/10.1111/jam.13302
-
The candidatus liberibacter-host interface: Insights into pathogenesis mechanisms and disease controlAnnual Review of Phytopathology 55:451–482.https://doi.org/10.1146/annurev-phyto-080516-035513
-
Lipid homeostasis is essential for oogenesis and embryogenesis in the silkworm, Bombyx moriCellular and Molecular Life Sciences 81:127.https://doi.org/10.1007/s00018-024-05173-8
Article and author information
Author details
Publication history
- Version of Record published: April 11, 2024 (version 1)
Copyright
© 2024, Zhang and An
This article is distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use and redistribution provided that the original author and source are credited.
Metrics
-
- 227
- views
-
- 28
- downloads
-
- 0
- citations
Views, downloads and citations are aggregated across all versions of this paper published by eLife.
Download links
Downloads (link to download the article as PDF)
Open citations (links to open the citations from this article in various online reference manager services)
Cite this article (links to download the citations from this article in formats compatible with various reference manager tools)
Further reading
-
- Ecology
- Evolutionary Biology
Seasonal animal dormancy is widely interpreted as a physiological response for surviving energetic challenges during the harshest times of the year (the physiological constraint hypothesis). However, there are other mutually non-exclusive hypotheses to explain the timing of animal dormancy, that is, entry into and emergence from hibernation (i.e. dormancy phenology). Survival advantages of dormancy that have been proposed are reduced risks of predation and competition (the ‘life-history’ hypothesis), but comparative tests across animal species are few. Using the phylogenetic comparative method applied to more than 20 hibernating mammalian species, we found support for both hypotheses as explanations for the phenology of dormancy. In accordance with the life-history hypotheses, sex differences in hibernation emergence and immergence were favored by the sex difference in reproductive effort. In addition, physiological constraint may influence the trade-off between survival and reproduction such that low temperatures and precipitation, as well as smaller body mass, influence sex differences in phenology. We also compiled initial evidence that ectotherm dormancy may be (1) less temperature dependent than previously thought and (2) associated with trade-offs consistent with the life-history hypothesis. Thus, dormancy during non-life-threatening periods that are unfavorable for reproduction may be more widespread than previously thought.
-
- Ecology
Declines in biodiversity generated by anthropogenic stressors at both species and population levels can alter emergent processes instrumental to ecosystem function and resilience. As such, understanding the role of biodiversity in ecosystem function and its response to climate perturbation is increasingly important, especially in tropical systems where responses to changes in biodiversity are less predictable and more challenging to assess experimentally. Using large-scale transplant experiments conducted at five neotropical sites, we documented the impacts of changes in intraspecific and interspecific plant richness in the genus Piper on insect herbivory, insect richness, and ecosystem resilience to perturbations in water availability. We found that reductions of both intraspecific and interspecific Piper diversity had measurable and site-specific effects on herbivory, herbivorous insect richness, and plant mortality. The responses of these ecosystem-relevant processes to reduced intraspecific Piper richness were often similar in magnitude to the effects of reduced interspecific richness. Increased water availability reduced herbivory by 4.2% overall, and the response of herbivorous insect richness and herbivory to water availability were altered by both intra- and interspecific richness in a site-dependent manner. Our results underscore the role of intraspecific and interspecific richness as foundations of ecosystem function and the importance of community and location-specific contingencies in controlling function in complex tropical systems.






