Peptidoglycan sensing by octopaminergic neurons modulates Drosophila oviposition
Figures
PGN-mediated NF-κB pathway activation decreases female oviposition.
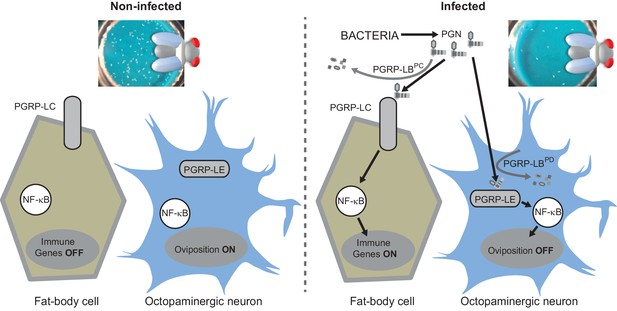
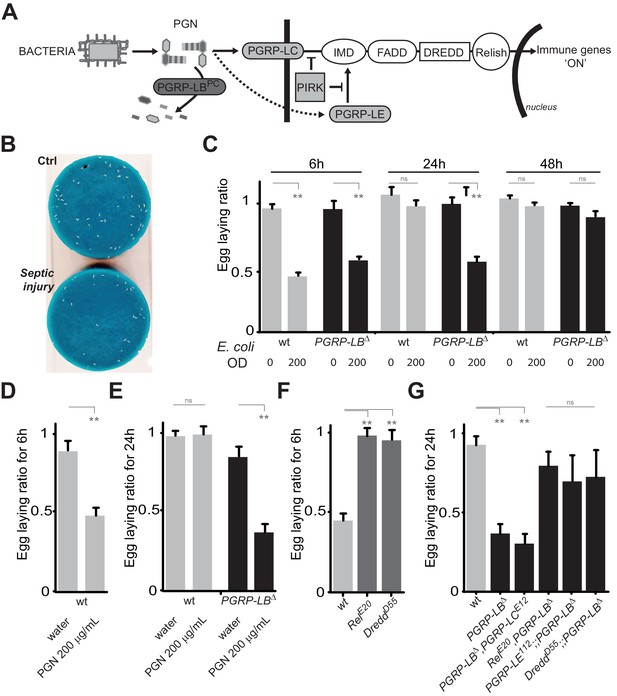
(A) Schematic representation of the Drosophila IMD pathway. Peptidoglycan (PGN) released by bacteria is recognized at the cell membrane by PGRP-LC or inside the cell by PGRP-LE. This PGRP/PGN interaction triggers via IMD, FADD, DREDD, nuclear translocation of Relish. Extracellular PGRP with amidase activity, such as PGRP-LBPC, dampens this signaling by degrading PGN. (B) Septic injury impairs egg-laying capacity in wild-type females. Pictures of fly tubes seen from the top. The blue dye is used to facilitate quantification of the eggs that appear as white dots. (C) Septic injury transiently impairs egg-laying capacity in wild type and in PGRP-LB mutant females. The egg-laying ratio for a time window (6 hr or 24 hr) corresponds to the number of eggs laid by a female after infection over the number of eggs laid by control female of the same genotype. (D and E) Injection of highly purified PGN in wt (D, E) and PGRP-LB mutants (E) is sufficient to reduce egg laying. (F) Mutation in Relish or in Dredd is preventing egg laying decrease post-infection. (G) Epistatic analyses showing that mutations in PGRP-LE, NF-κB or Dredd, but not in PGRP-LC are rescuing PGRP-LB mutant phenotype. For C, D, E, F and G; shown is the average egg-laying ratio ± SEM from at least two independent trials with at least 20 females per genotype and condition used. * indicates p<0.01; ** indicates p<0.001; n.s. indicates p>0.05, unpaired two-tailed Mann-Whitney test versus indicated controls.
Infection by bacteria decreases female oviposition.
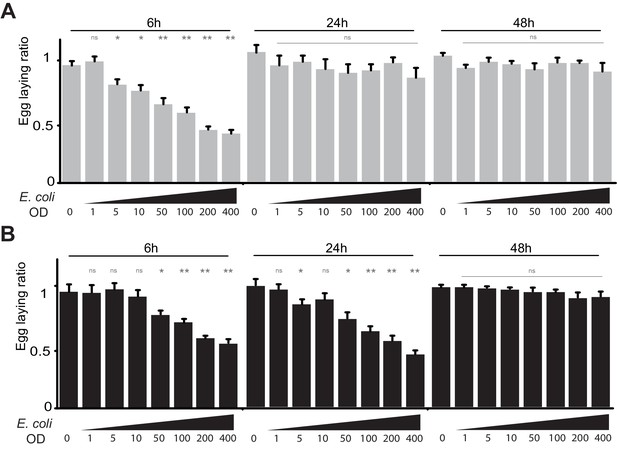
(A) The effect of bacterial infection on egg laying is dose-dependent and reversible. Egg-laying ratio of wt (A) and PGRP-LB mutants (B) quantified after septic injury with various E. coli concentrations. (B) The oviposition drop is not due to the cuticle injury since it is not observed after an aseptic injury (OD 0). For A and B; shown is the average egg-laying ratio ± SEM from at least two independent trials with at least 20 females per genotype and condition used. * indicates p<0.01; ** indicates p<0.001; n.s. indicates p>0.05, unpaired two-tailed Mann-Whitney test versus sterile injury.
IMD pathway overactivation leads to an exacerbated oviposition drop following exposure to bacteria.
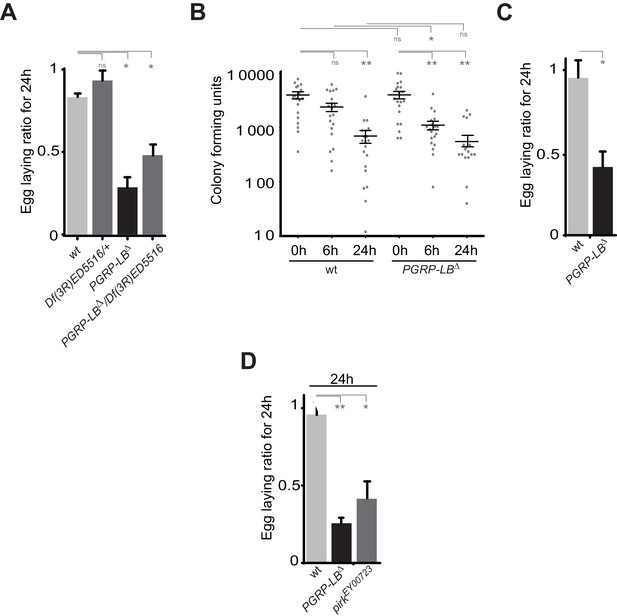
(A) The exacerbated oviposition drop following bacterial infection in PGRP-LB mutant is also observed in transheterozygote PGRP-LB mutants. (B) The exacerbated phenotype observed in PGRP-LB mutant compared to controls is not due to different bacterial loads within genotypes. Following septic injury with E. coli at OD 200, colony-forming units were quantified from individual flies at various time points. (C) Feeding PGRP-LB mutants with bacteria induces an egg-laying drop. When adult flies were orally infected with the entomopathogenic bacteria Erwinia carotovora carotovora (Ecc), a robust egg-laying drop was observed in PGRP-LB mutant flies. (D). As for PGRP-LB, mutation in pirk leads to an exacerbated oviposition drop following septic injury. For A, C, and D; shown is the average egg-laying ratio ± SEM from at least two independent trials with at least 20 females per genotype and condition used. * indicates p<0.01; ** indicates p<0.001, n.s. indicates p>0.05, unpaired two-tailed Mann-Whitney test versus indicated control.
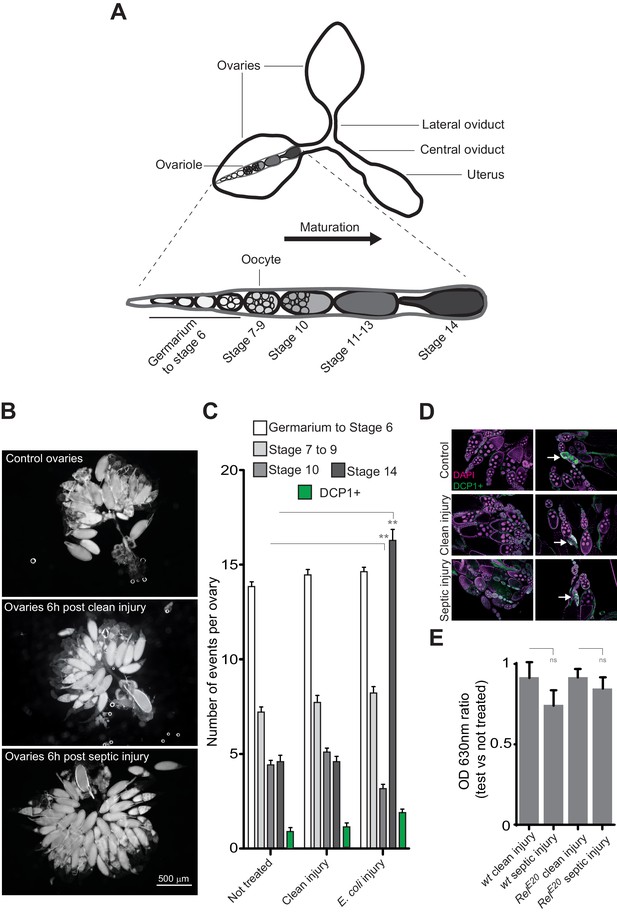
Bacterial infection provokes an accumulation of mature oocytes in female ovaries.
(A) Diagram representing ovaries containing ovarioles and oocytes within a single ovariole. (B) Following septic injury, stage 14 oocytes accumulate in ovaries. Pictures of opened ovaries with oocytes of various stages depending on the treatment. At the indicated time, female ovaries were dissected in PBS and opened. (C) 6 hr following treatment, wt female ovaries were dissected and the number of oocytes of the different stages was quantified. Apoptotic events were quantified using immunostaining against activated caspase (DCP1+). (D) Representative pictures of ovaries stained with DAPI and with an antibody against activated DCP1 following various treatments. Apoptotic events are indicated with a white arrow. (E) Food intake is not impaired 6 hr post-septic injury. The ratio of blue dye in intestines and crops of infected versus non-infected females was quantified. For C, shown is the average number of events per ovary ± SEM from three independent experiments with at least 20 ovaries per condition used. For E, shown is average OD ratio ± SEM from at least two independent trials with at least 20 dissected intestines per genotype and condition used. ** indicates p<0.001, Tukey’s multiple comparison test.
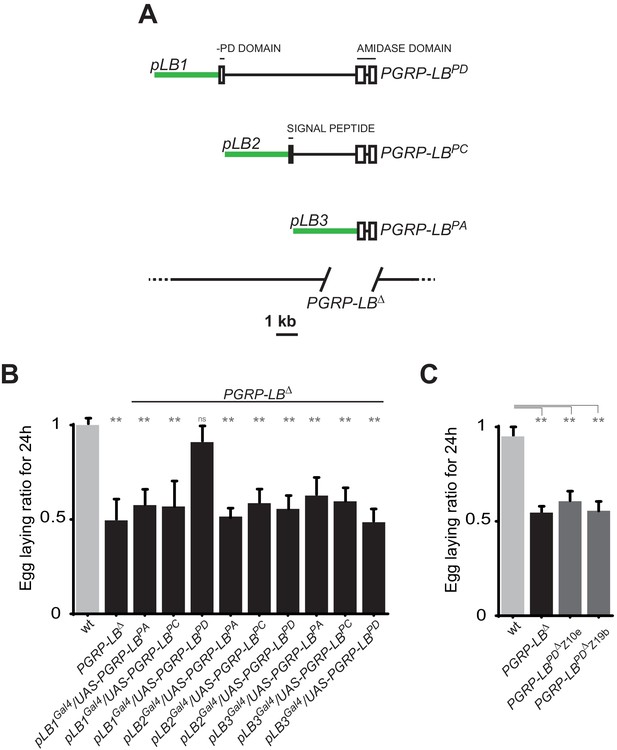
One specific PGRP-LB isoform controls oviposition.
(A) PGRP-LB locus organization. Three PGRP-LB isoforms are produced from the locus. The green lines represent the cloned fragments used to generate the Gal4 constructs. (B) Egg-laying ratio of PGRP-LB females carrying various Gal4 drivers (pLB1Gal4, pLB2 Gal4, pLB3 Gal4) and UAS constructs allowing the overexpression of the three different PGRP-LB isoforms (PGRP-LBPA, PGRP-LBPC, PGRP-LBPD). A single Gal4-UAS combination (pLB1Gal4/UAS–PGRP-LBPD) is rescuing the egg-laying drop seen in infected PGRP-LB mutant females. (C) Egg-laying ratio of PGRP-LB mutant females and CRISPR-Cas9-generated PGRP-LBPD only mutants. For B and C: shown is the average egg-laying ratio ± SEM from at least two independent trials with at least 20 females per genotype and condition used. * indicates p<0.01; ** indicates p<0.001; n.s. indicates p>0.05, unpaired two-tailed Mann Whitney test versus wt animals.
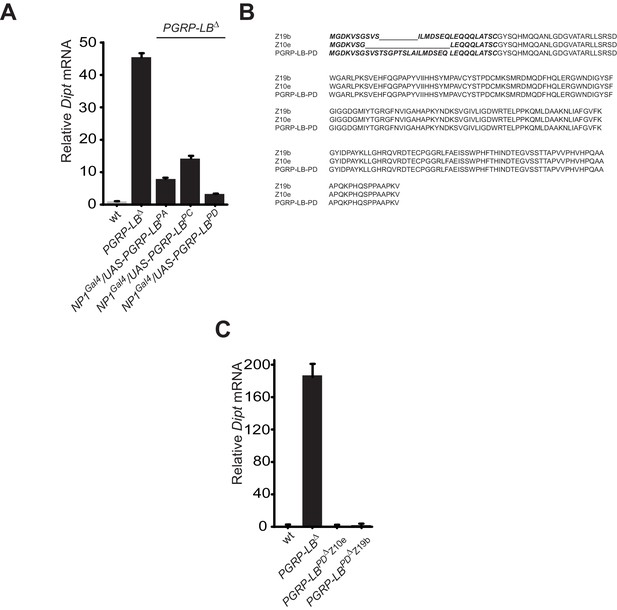
While all PGRP-LB isoforms possess amidase activity, PGRP-LBPD isoform is not required for the negative regulation of the systemic immune response.
(A) Quantification of Diptericin mRNA in wt and PGRP-LB mutant guts expressing the different PGRP-LB isoforms under the control of the gut specific Gal4 driver, NP1Gal4 and orally infected with Ecc15. (B) Amino acid sequence alignment showing the deletions in the PGRP-LBPD isoform-specific mutants generated. In bold are the amino acids specific of the PGRP-LBPD isoform. (C) Quantification of Diptericin mRNA in wt, PGRP-LB mutant and PGRP-LBPD isoform-specific mutants fat body orally infected with Ecc15.
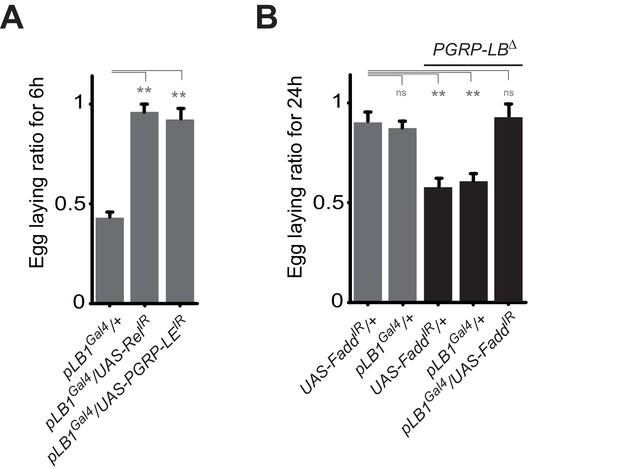
A functional NF-κB pathway is required in pLB1 cells to modulate egg-laying post-infection.
(A) Egg-laying ratio of wt females in which the IMD pathway has been inactivated in pLB1 cells only (pLB1Gal4/UAS-RelIR and pLB1Gal4/UAS-PGRP-LEIR)(B) Egg-laying ratio of PGRP-LB mutant females in which the IMD pathway has been inactivated in pLB1 cells only (pLB1Gal4/UAS-FaddIR). For A and B; shown is the average egg-laying ratio ± SEM from at least two independent trials with at least 20 females per genotype and condition used. * indicates p<0.01; ** indicates p<0.001; n.s. indicates p>0.05, unpaired two-tailed Mann-Whitney test versus controls.
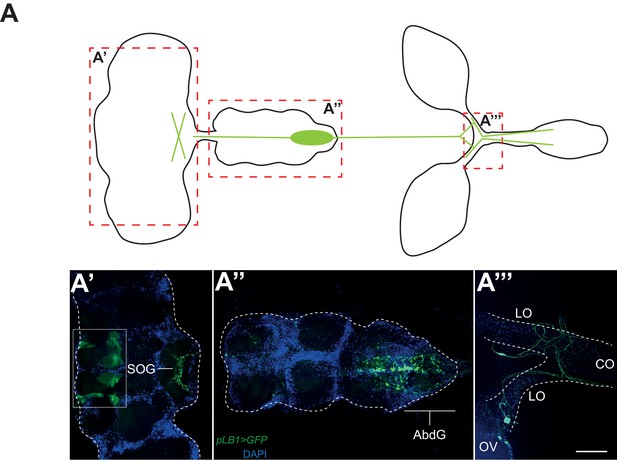
pLB1 is expressed in cells of the nervous system.
(A) Diagram summarizing pLB1 expression pattern (green lines) in the brain (A’), the Ventral Nerve Cord (VNC) (A’’) and the ovaries (A’’’). Expression pattern of pLB1QF/QUAS-GFP (pLB1>GFP) in females. In the brain (A’), pLB1 is expressed in the Sub Oesophageal Ganglion (SOG). The area in the box corresponds to unspecific transgene expression seen in the QUAS-GFP strain alone. In A’’, the expression is restricted to the Abdominal Ganglia (AbdG) that corresponds to the posterior tip of the VNC. In A’’’, lateral oviducts (LO) connecting ovaries (OV) to the central oviduct (CO) are shown. Staining is also observed in other tissues such as pericardiac cells, some enterocytes (data not shown). The scale bar corresponds to 50 µm.
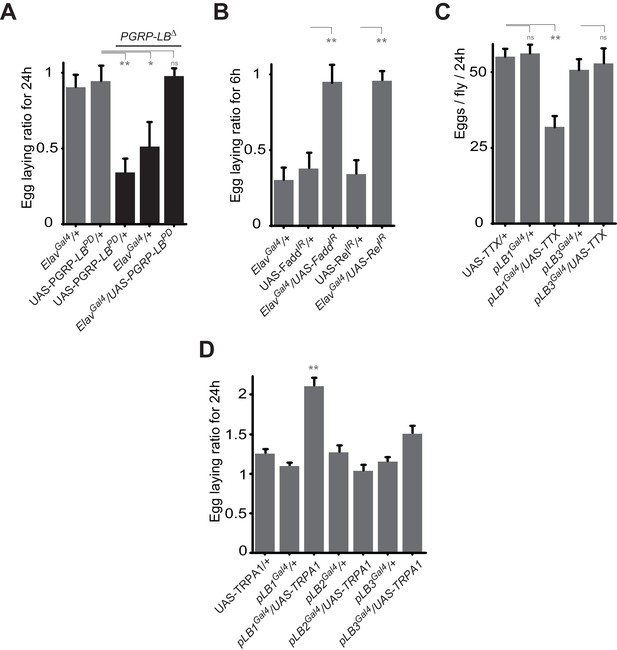
Some pLB1 cells are neurons controlling oviposition via NF-κB.
(A) Egg-laying ratio of PGRP-LB mutant females in which the pan-neuronal Gal4 drivers (Elav Gal4) is used to drive expression of the PGRP-LBPD isoform. Expression of the PGRP-LBPD isoform in neurons only is sufficient to rescue PGRP-LB mutant phenotype following septic injury. (B) RNAi-mediated inactivation of the NF-κB pathway prevents egg-laying drop post-infection. (C) Expression of Tetanus Toxin (TTX) in pLB1, but not in pLB3-positive cells, is sufficient to decrease egg laying in non-infected mated females. pLB2Gal4/UAS-TTX flies are not viable (data not shown). (D) Expression of the transient receptor potential cation channel (TRPA1) in pLB1 but neither in pLB2 nor pLB3-positive cells stimulates egg laying in non-infected mated females. For A and B; shown is the average egg-laying ratio ± SEM from at least two independent trials with at least 20 females per genotype and condition used. For C; shown is the average number of eggs laid per fly per 24 hr ± SEM from at least two independent trials with at least 20 females per genotype and condition used. For D, shown is the average egg-laying ratio, the number of eggs laid by females raised at 29°C (permissive temperature for TPRA1) over the number of eggs laid at 23°C (restrictive), ± SEM from at least two independent trials with at least 20 females per genotype and condition used. * indicates p<0.01; ** indicates p<0.001; n.s. indicates p>0.05, unpaired two-tailed Mann-Whitney test versus controls (for A, B and C) and Dunn’s multiple comparison test (for D).
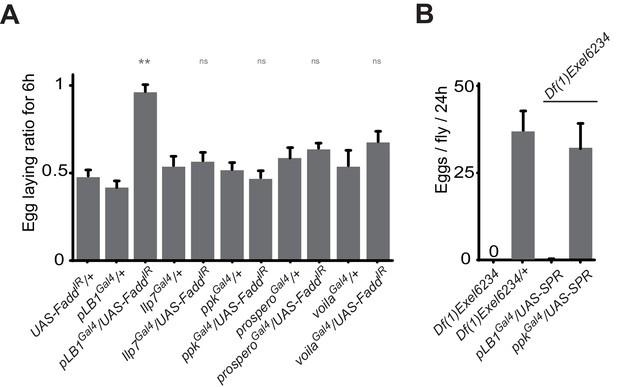
ppk, Ilp7, prospero or voila-positive cells do no regulate female egg laying after infection.
(A) Egg-laying ratio of wt mated females in which the IMD pathway has been specifically inactivated via UAS-FaddIR ectopic expression in ppk, Ilp7, prospero or voila-positive cells. (B) While expressing the SPR cDNA in ppk-positive cells fully rescue egg-laying defect seen in SPR-deficient females, this is not the case if SPR cDNA is expressed in pLB1-positive cells. For A; shown is the average egg-laying ratio ± SEM from at least two independent trials with at least 20 females per genotype and condition used. For B; shown is the average number of eggs laid per fly per 24 hr ± SEM with at least 10 females per genotype and condition used, the graph is representative of two independent experiments. ** indicates p<0.001; n.s. indicates p>0.05, unpaired two-tailed Mann-Whitney test versus controls.
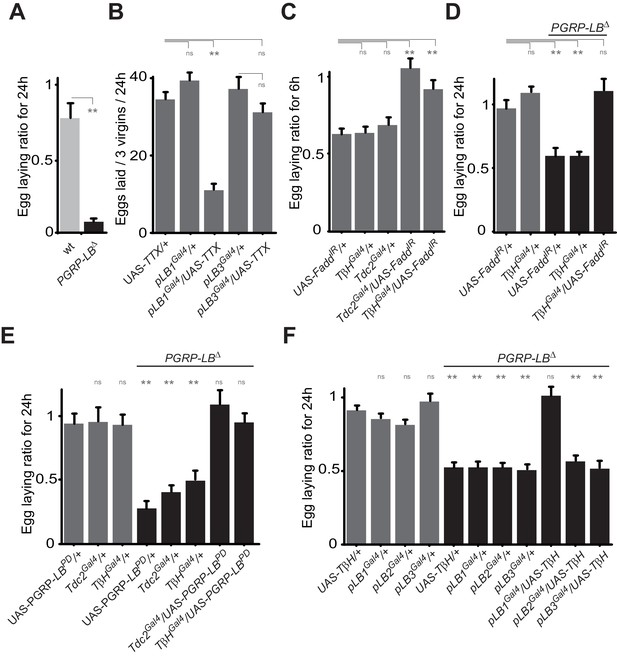
Bacteria modulate egg-laying behavior via the octopamine pathway.
(A) Septic injury reduces egg laying in PGRP-LB virgin females. Egg-laying ratio post-septic injury of wt and PGRP-LB mutant virgin females. (B) Functional inactivation of pLB1, but not of pLB3-positive cells, by UAS-TTX blocks egg laying in virgin females. Total eggs laid by wild-type virgins in which the TTX is expressed in pLB1 or pLB3 cells. (C and D) Egg-laying ratio of wt (C) or PGRP-LB mutant mated females (D) in which the IMD pathway has been specifically inactivated via UAS-FaddIR ectopic expression in TβH or Tdc2-positive cells. (E) Restoring PGRP-LBPD expression in cells that produce the enzymes required to synthesize octopamine (tdc2 and TβH) fully rescues the PGRP-LB mutant phenotype following septic injury. Egg-laying ratio of PGRP-LB mutant females in which the tdc2 Gal4 and TβHGal4 drivers are used to overexpress the PGRP-LBPD isoform. (F) Providing an excess of TβH in pLB1 cells is sufficient to rescue PGRP-LB mutant phenotypes. Egg-laying ratio of PGRP-LB mutant mated females in which the TβH level has been increased in pLB1, pLB2 or pLB3 cells. For A, C, D, E and F; shown is the average egg-laying ratio ± SEM from at least two independent trials with at least 20 females per genotype and condition used. For (B); shown is the average number of eggs laid per three virgins per 24 hr ± SEM from at least two independent trials with at least 20 females per genotype and condition used. * indicates p<0.01; ** indicates p<0.001; n.s. indicates p>0.05, unpaired two-tailed Mann-Whitney test versus indicated controls for A, B, C and D and versus UAS in wt background for E and F.
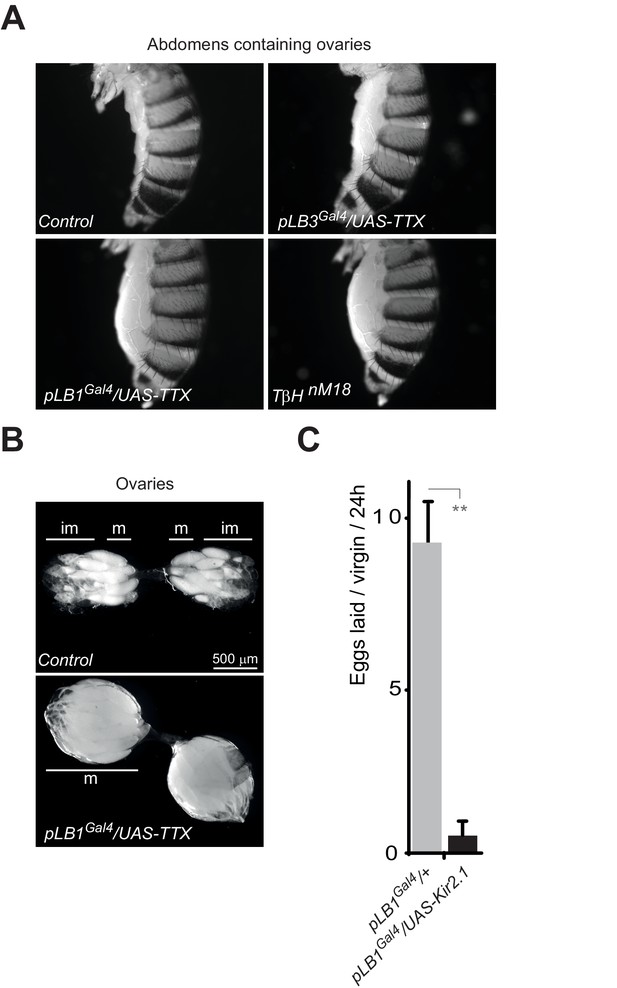
Inactivation of pLB1 cells phenocopies TβH mutant.
(A) Functional inactivation of pLB1 cells using the inwardly-rectifying K+ channel Kir (2.1b) that inhibits neuronal activity blocks egg laying in virgin females. (B) Ovaries of pLB1Gal4/UAS-TTX virgin females are packed with fully mature (m) oocytes, in contrast to controls (pLB1Gal4) in which ovarioles contain both immature (im) and mature oocytes. (C) Functional inactivation of pLB1 cells phenocopies mutations in enzymes required to produce octopamine. Abdomens of pLB1Gal4/UAS-TTX virgin females are filled with ovaries packed with fully mature oocytes and resembles TβHnM18 mutant females. This is not the case in controls (pLB1Gal4) or in pLB3Gal4/UAS-TTX females. For A; shown is the average number of eggs laid per virgin per 24 hr ± SEM from at least two independent trials with at least 20 females per genotype and condition used. ** indicates p<0.001, unpaired two-tailed Mann-Whitney test versus indicated control.