A toolbox of IgG subclass-switched recombinant monoclonal antibodies for enhanced multiplex immunolabeling of brain
Figures
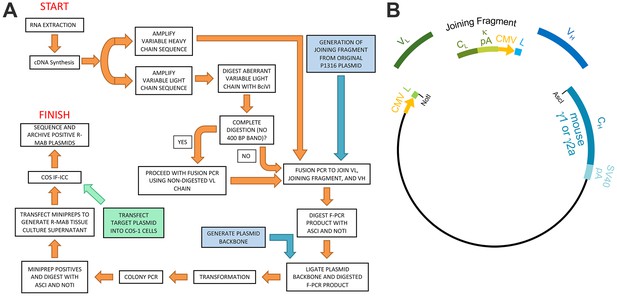
Schematic representation of the R-mAb pipeline.
(A) Schematic of cloning, expression and validation pipeline. Orange steps involve VH and VL regions of individual hybridomas, blue steps involve steps involving backbone components, and green step involves expression of target for R-mAb validation. (B) Schematic shows the separate elements of the R-mAb expression plasmid involved in coexpression of light (green) and heavy (blue) chains as driven by two CMV promoters (orange). Hybridoma-derived VL and VH domain PCR products are fused to a joining fragment comprising a κ light chain constant domain (CL) and the κ light chain polyA tail sequences (κ pA), a CMV promoter for heavy chain expression, and an ER signal/leader sequence (L) for translocation of the heavy chain across the ER membrane. PCR-mediated fusion of these three elements is followed by their insertion into the p1316 plasmid that contains an upstream CMV promoter for light chain expression, and an ER signal/leader sequence (L) for translocation of the light chain across the ER membrane. Downstream of the insert is a heavy chain constant domain (CH) that is either γ1 or γ2a depending on the plasmid, followed by the SV40 polyA tail (SV40 pA).
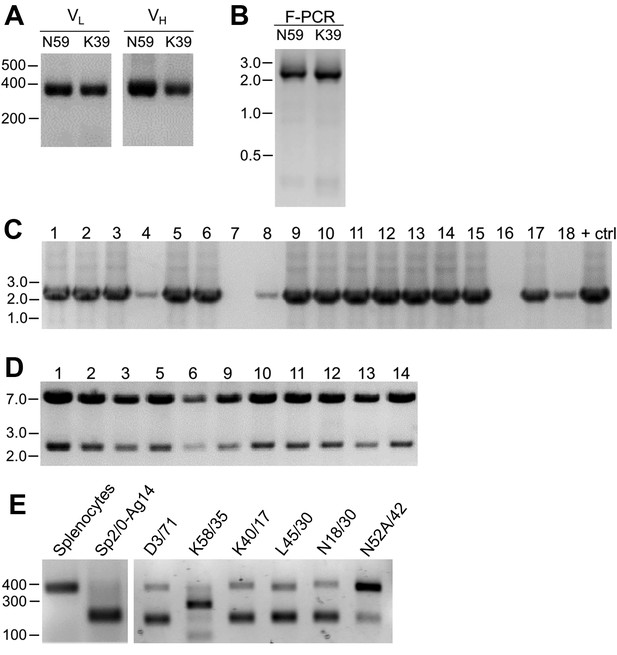
Cloning of VL and VH domain sequences from hybridomas into the R-mAb expression plasmid.
(A) Agarose gel analysis of VL and VH domain PCR products amplified from cDNA synthesized from RNA extracted from the N59/36 (anti-NR2B/GRIN2B) and K39/25 (anti-Kv2.1/KCNB1) hybridomas. The expected size of mouse IgG VL and VH domains is ≈360 bp. (B) Agarose gel analysis of VH and digested VL fragments joined by fusion PCR (F-PCR) to the P1316-derived joining fragment to create a dual IgG chain cassette. (C) Agarose gel analysis of colony PCR samples of transformants from the N59/36 R-mAb project. (D) Agarose gel analysis of products of restriction enzyme digestion of N59/36 plasmid DNA with NotI and AscI. The plasmid backbone is seven kbp, and the intact insert comprising the VL and VH domains and the intervening joining fragment is 2.4 kbp. (E) Agarose gel analysis of PCR products of VL domain cDNA synthesized from RNA extracted from mouse splenocytes, the fusion partner Sp2/0-Ag14, and various hybridomas after digestion with the BciVI restriction enzyme to cleave the Sp2/0-Ag14-derived aberrant light chain product. The intact VL domains are ≈360 bp, and the digested aberrant light chains ≈180 bp.
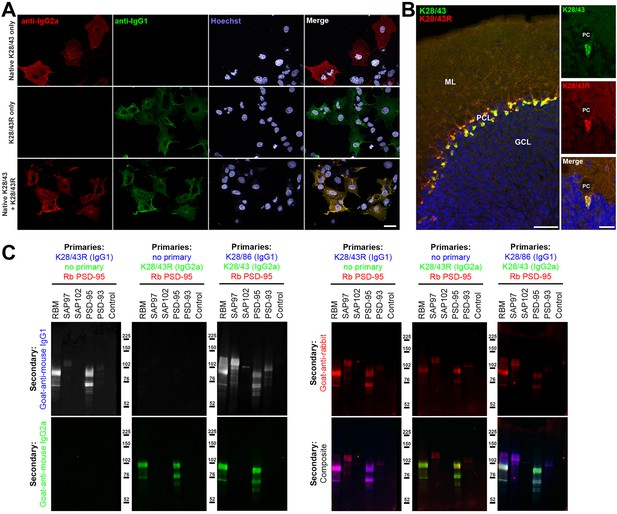
Validation of subclass-switched anti-PSD-95 K28/43R R-mAb.
(A) Validation of the K28/43R R-mAb in heterologous cells. COS-1 cells transiently transfected to express human PSD-95 in a subset of cells were immunolabeled with K28/43 mAb (IgG2a) alone (top row), K28/43R R-mAb (IgG1) alone (middle row), or K28/43 mAb plus K28/43R R-mAb (bottom row). Immunolabeling in all samples was detected with a cocktail of anti-mouse IgG2a (red, for the K28/43 mAb) and anti-mouse IgG1 (green, for the K28/43R R-mAb) subclass-specific Alexa Fluor conjugated secondary antibodies. Labeling in blue is for the DNA-specific dye Hoechst 33258 and shows nuclei of both transfected and untransfected cells. Scale bar in the lower right merged panel = 30 µm and holds for all panels in A. (B) Validation of the K28/43R R-mAb in brain sections. A brain section from an adult rat was immunolabeled with K28/43 mAb plus K28/43R R-mAb and immunolabeling detected with a cocktail of anti-mouse IgG2a (red, for K28/43 mAb) and anti-mouse IgG1 (green, for K28/43R R-mAb) subclass-specific Alexa Fluor conjugated secondary antibodies. Cell nuclei are labeled with the DNA-specific dye Hoechst 33258 (blue). The region of interest shown is from cerebellar cortex. Scale bar in the left panel = 100 µm, and in the right merged panel = 30 µm. (C) Immunoblots against brain membranes and COS cell lysates over-expressing various members of the MAGUK superfamily of scaffolding proteins. To confirm expression of MAGUK proteins, immunoblots were probed with rabbit anti-PSD-95 (red). K28/86 is an anti-MAGUK mAb. Primary antibodies were detected with the appropriate combinations of fluorescently labeled species-specific anti-rabbit and subclass-specific anti-mouse IgG secondary Abs as indicated. Control indicates COS cells transfected with an empty vector.
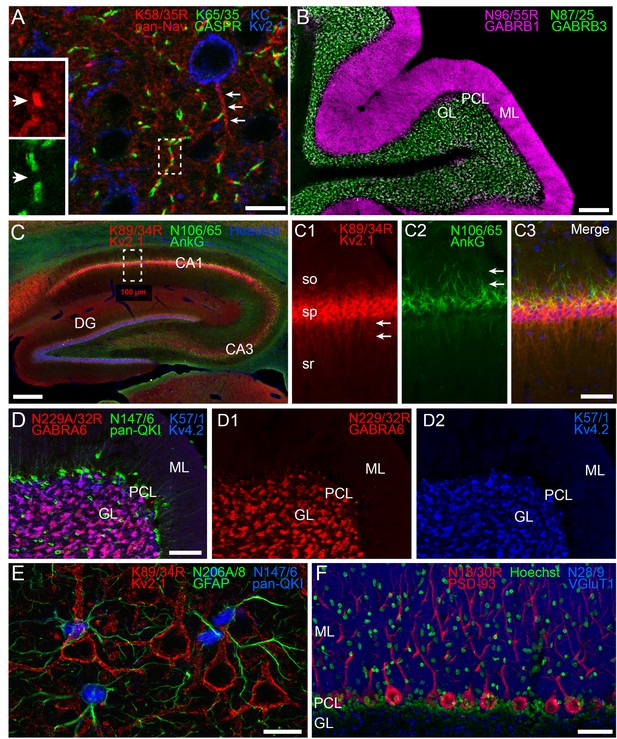
Multiplex immunolabeling with subclass-switched recombinant antibodies in adult rat brain.
(A) A section from neocortex labeled with anti-pan-Nav R-mAb K58/35R (IgG2a, red) at nodes of Ranvier and AIS (arrows), anti-CASPR mAb K65/35 (IgG1, green) at paranodes, and anti-Kv2.1 rabbit polyclonal (KC) antibody (blue) on somata and proximal dendrites. Scale bar = 150 μm. Insets (dashed box) show details of labeling for pan-Nav (red) and CASPR (green) at the node and paranodes (arrows), respectively, at a single node of Ranvier as indicated by box in main panel. (B) A section through cerebellum showing labeling with anti-GABA-AR β1 R-mAb N96/55R (IgG2a, magenta) in the molecular layer (ML), and anti-GABA-AR β3 mAb N87/25 (IgG1, green) in the granule cell layer (GL). PCL = Purkinje cell layer. Scale bar = 150 μm. (C) A section through hippocampus labeled with anti-Kv2.1 R-mAb 89/34R (IgG2a, red) on somata and proximal dendrites, anti-AnkyrinG mAb N106/65 (IgG2b, green) on AIS, and nuclear stain Hoechst 33258 (blue). Scale bar = 150 μm. Panels C1-C3 show magnified details of labeling for pan-Kv2.1 (red) on somata and proximal dendrites (arrows in C1), and anti-AnkyrinG (green) on AIS (arrows in C2). Scale bar = 50 μm (C1–C3). (D) A section through cerebellum labelled with anti-GABA-AR α6 R-mAb K229A/32R (IgG2a, red) in the granule cell layer (GL), anti-pan-QKI mAb N147/6 (IgG2b, green) labeling glial cells in/near the Purkinje cell layer (PCL), and anti-Kv4.2 mAb K57/1 (IgG1, blue) labeling the granule cell layer (GL). Scale bar = 30 μm. (E) A section from neocortex labelled with anti-Kv2.1 R-mAb 89/34R (IgG2a, red) on somata and proximal dendrites of neurons, and anti-GFAP mAb N206A/8 (IgG1, green) and anti-pan-QKI mAb N147/6 (IgG2b, blue) labeling glial cell processes and cell bodies respectively. Scale bar = 15 μm. (F) A section through cerebellum showing labeling with anti-PSD-93 R-mAb N18/30R (IgG2a, red) in the cell bodies and dendrites of Purkinje cells, the nuclear stain Hoechst 33258 (green) and anti-VGluT1 mAb N28/9 (IgG1, blue) in the molecular layer (ML). PCL = Purkinje cell layer. Scale bar = 10 μm.
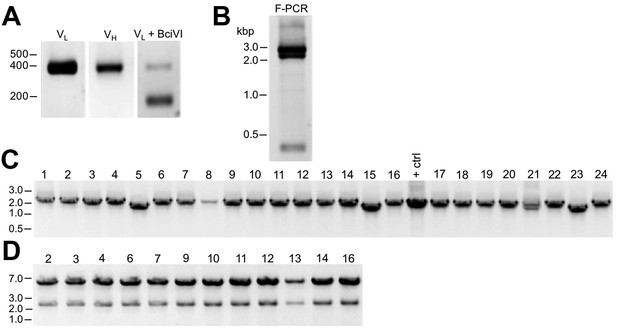
Cloning of anti-Kv2.1 D3/71 VL and VH domain cDNAs from a nonviable hybridoma.
(A) Agarose gel analysis of PCR amplified VL and VH domains from cDNA synthesized from RNA extracted from the non-viable D3/71 hybridoma. The panel to the right shows the VL after digestion with the BciVI restriction enzyme to cleave the Sp2/0-Ag14-derived aberrant light chain product. The expected size of mouse IgG VL and VH domains is ≈360 bp, and of the cleaved aberrant VL domain is ≈180 bp. (B) Agarose gel analysis of D3/71 VH and digested VL fragments joined by fusion PCR (F-PCR) to the P1316 joining fragment to create a dual IgG chain cassette. (C) Agarose gel analysis of colony PCR samples of transformants from the of D3/71 R-mAb project. (D) Agarose gel analysis of products of restriction enzyme digestion of D3/71 plasmid DNA with NotI and AscI. The plasmid backbone is seven kbp, and the intact insert comprising the VL and VH domains and the intervening joining fragment is 2.4 kbp.
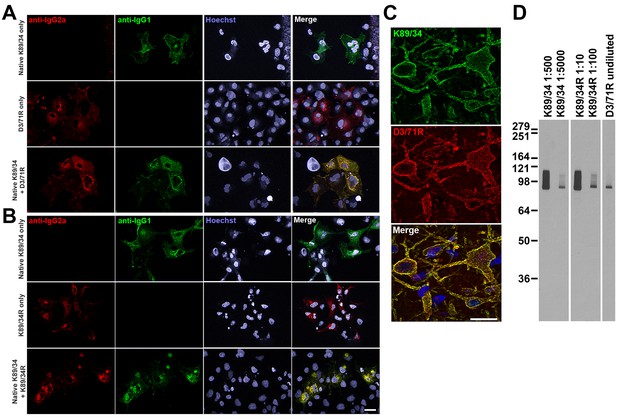
Recovery of a functional anti-Kv2.1 D3/71R R-mAb from nonviable hybridomas.
(A) Validation of the D3/71R R-mAb in heterologous cells. COS-1 cells transiently transfected to express rat Kv2.1 in a subset of cells were immunolabeled with K89/34 mAb (IgG1) alone (top row), D3/71R R-mAb (IgG2a) alone (middle row), or K89/34 mAb plus D3/71R R-mAb (bottom row). Immunolabeling in all samples was detected with a cocktail of anti-mouse IgG1 (green, for the K89/34 mAb) and anti-mouse IgG2a (red, for the D3/71R R-mAb) subclass-specific Alexa Fluor conjugated secondary antibodies. (B) Validation of the subclass-switched K89/34R R-mAb in heterologous cells. COS-1 cells transiently transfected to express rat Kv2.1 in a subset of cells were immunolabeled with K89/34 mAb (IgG1) alone (top row), K89/34R R-mAb (IgG2a) alone (middle row), or K89/34 mAb plus K89/34R R-mAb (bottom row). Immunolabeling in all samples was detected with a cocktail of anti-mouse IgG1 (green, for the K89/34 mAb) and anti-mouse IgG2a (red, for the K89/34R R-mAb) subclass-specific Alexa Fluor conjugated secondary antibodies. Labeling in blue in panels A and B is for the DNA-specific dye Hoechst 33258 and shows nuclei of both transfected and untransfected cells. Scale bar in the lower right merged panel = 30 µm and holds for all panels in A and B. (C) Validation of the D3/71R R-mAb in brain sections. A brain section from an adult rat was immunolabeled with K89/34 mAb plus D3/71 R-mAb and the immunolabeling detected with a cocktail of anti-mouse IgG1 (green, for the K89/34 mAb) and anti-mouse IgG2a (red, for the D3/71R R-mAb) subclass-specific Alexa Fluor conjugated secondary antibodies. Cell nuclei are labeled with the DNA-specific dye Hoechst 33258 (blue). Region of interest shown is from neocortex. Scale bar = 30 µm. (D) Strip immunoblots on a crude rat brain membrane fraction immunolabeled with the K89/34 mAb, the K89/34R R-mAb, and the D3/71 R-mAb as indicated. Immunolabeling was detected on autoradiography film after treatment of strip immunoblots with HRP-conjugated anti-mouse IgG-specific secondary antibody and ECL.
Tables
Aberrant VL sequences remaining after BciVI digestion.
Table delineates for specific mAb cloning projects the total number of clones sequenced, and the number of sequenced clones with VL chains corresponding to the Sp2/0-Ag14 hybridoma-derived aberrant VL transcript.
| mAb | Number of clones sequenced | Number of clones with aberrant chain | % with aberrant chain |
|---|---|---|---|
| K7/45 | 5 | 0 | 0 |
| K9/40 | 7 | 2 | 28.6 |
| K13/31 | 10 | 0 | 0 |
| K14/16.2 | 2 | 0 | 0 |
| K14/16.2.1 | 9 | 2 | 22.2 |
| K17/70 | 6 | 1 | 16.7 |
| K28/86 | 3 | 0 | 0 |
| K36/15 | 6 | 1 | 16.7 |
| K74/71 | 6 | 0 | 0 |
| K75/41 | 3 | 0 | 0 |
| K87A/10 | 3 | 0 | 0 |
| L6/60 | 3 | 0 | 0 |
| L21/32 | 4 | 0 | 0 |
| L23/27 | 6 | 0 | 0 |
| L61/14 | 7 | 1 | 14.3 |
| N59/20 | 2 | 2 | 100 |
| N70/28 | 1 | 0 | 0 |
| N86/8 | 8 | 2 | 25.0 |
| N86/38 | 22 | 0 | 0 |
| N100/13 | 6 | 0 | 0 |
| N103/31 | 4 | 1 | 25.0 |
| N103/39 | 5 | 0 | 0 |
| N105/13 | 5 | 0 | 0 |
| N106/36 | 6 | 0 | 0 |
| N116/14 | 3 | 0 | 0 |
| N297/59 | 7 | 0 | 0 |
| Total | 149 | 12 | 8.1 |
| Reagent type (species) or resource | Designation | Source or reference | Identifiers | Additional information |
|---|---|---|---|---|
| Cell line (Cercopithecus aethiops) | COS-1 | ATCC Cat No CRL-1650; PMID: 6260373 | RRID:CVCL_0223 | |
| Antibody | numerous | See Table 2, Supplementary file 1, 2 | ||
| Recombinant DNA reagent | p1316 plasmid | PMID: 20525357 | ||
| Software | Photoshop | Adobe Systems | RRID:SCR_014199 | |
| Software | Axiovision | Carl Zeiss MicroImaging | RRID:SCR_002677 | |
| Software | Fiji | PMID: 22743772 | RRID:SCR_002285 |
Non-R-mAb antibodies used in this study.
Table lists Abs used in this study outside of the R-mAbs whose generation is described here. For each Ab the name, immunogen used in Ab generation, source and RRID number in the Antibody Registry, form and concentration/dilution used, and specific use in this paper is detailed.
| Antibody | Immunogen | Manufacturer information | Concentration/dilution used | Figures |
|---|---|---|---|---|
| KC | Synthetic peptide aa 837–853 of rat Kv2.1 | Rabbit pAb, In-house (Trimmer Laboratory), RRID:AB_2315767 | Affinity purified, 1:100 | 4 |
| PSD-95 | Fusion protein aa 77–299 of human PSD-95 | Rabbit pAb, In-house (Trimmer Laboratory), RRID:AB_2750832 | Affinity purified, 1:150 | 3 |
| K28/43 | Fusion protein aa 77–299 of human PSD-95 | Mouse IgG2a mAb, NeuroMab RRID:AB_10698024 | Tissue culture supernatant, 1:5 | 3 |
| K28/86 | Fusion protein aa 77–299 of human PSD-95 | Mouse IgG1 mAb, NeuroMab RRID:AB_10698179 | Tissue culture supernatant, 1:5 | 3 |
| K57/1 | Synthetic peptide aa 209–225 of human Kv4.2 | Mouse IgG1 mAb, NeuroMab RRID:AB_10672254 | Tissue culture supernatant, 1:5 | 4 |
| K65/35 | Fusion protein aa 1308–1381 of rat CASPR | Mouse IgG1 mAb, NeuroMab, RRID:AB_10671175 | Tissue culture supernatant, 1:5 | 4 |
| K89/34 | Synthetic peptide aa 837–853 of rat Kv2.1 | Mouse IgG1 mAb, NeuroMab RRID:AB_10672253 | Tissue culture supernatant, 1:10 | 6 |
| N28/9 | Fusion protein aa 492–560 of rat VGluT1 | Mouse IgG1 mAb, NeuroMab RRID:AB_10673111 | Tissue culture supernatant, 1:5 | 4 |
| N87/25 | Fusion protein aa 370–433 of mouse GABA-A-receptor β3 subunit | Mouse IgG1 mAb, NeuroMab RRID:AB_10673389 | Tissue culture supernatant, 1:5 | 4 |
| N106/65 | Full-length recombinant human Ankyrin-G | Mouse IgG2a mAb, NeuroMab RRID:AB_10673449 | Tissue culture supernatant, 1:5 | 4 |
| N147/6 | Fusion protein aa 1–341 (full-length) of human QKI-5 | Mouse IgG2b mAb, NeuroMab RRID:AB_10671658 | Tissue culture supernatant, 1:5 | 4 |
| N206A/8 | Synthetic peptide aa 411–422 of human GFAP | Mouse IgG1 mAb, NeuroMab RRID:AB_10672298 | Tissue culture supernatant, 1:5 | 4 |
Additional files
-
Supplementary file 1
Conversion efficiency of a selected subset of R-mAbs.
Table details for a set of 181 projects (130 successful, 51 unsuccesful) the number of colony PCR or restriction digest positive clones that were input into the COS-ICC validation assay, and the number and percentage of these that were positive in that assay.
- https://doi.org/10.7554/eLife.43322.011
-
Supplementary file 2
mAbs converted to validated R-mAbs.
Table lists the mAbs successfully converted to R-mAbs to date. For each mAb, the mAb clone number, the target protein, the mAb IgG subclass (in sentence case), the R-mAb IgG subclass (in upper case) and the original mAb and the cloned R-mAb RRID numbers in the Antibody Registry are detailed.
- https://doi.org/10.7554/eLife.43322.012
-
Transparent reporting form
- https://doi.org/10.7554/eLife.43322.013





